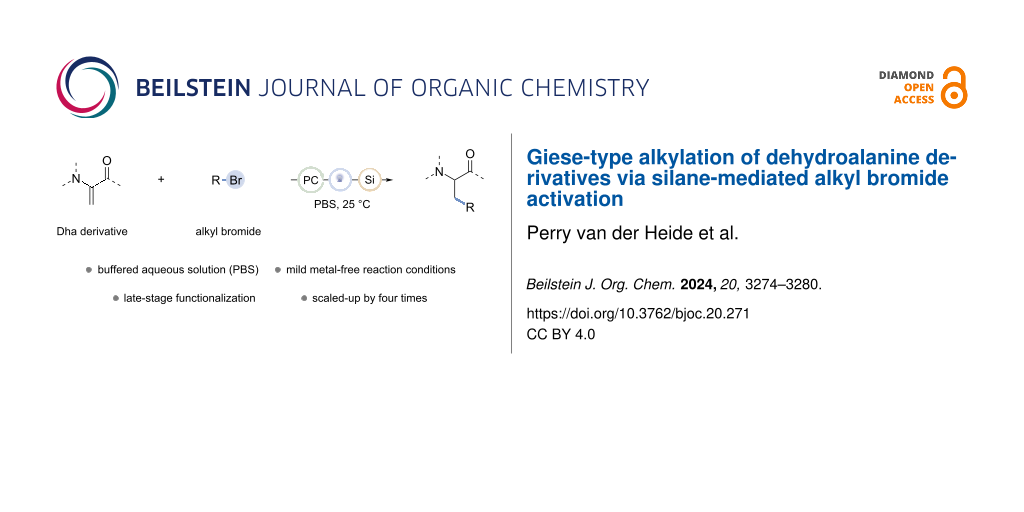
Abstract
The rising popularity of bioconjugate therapeutics has led to growing interest in late-stage functionalization (LSF) of peptide scaffolds. α,β-Unsaturated amino acids like dehydroalanine (Dha) derivatives have emerged as particularly useful structures, as the electron-deficient olefin moiety can engage in late-stage functionalization reactions, like a Giese-type reaction. Cheap and widely available building blocks like organohalides can be converted into alkyl radicals by means of photoinduced silane-mediated halogen-atom transfer (XAT) to offer a mild and straightforward methodology of alkylation. In this research, we present a metal-free strategy for the photochemical alkylation of dehydroalanine derivatives. Upon abstraction of a hydride from tris(trimethylsilyl)silane (TTMS) by an excited benzophenone derivative, the formed silane radical can undergo a XAT with an alkyl bromide to generate an alkyl radical. Consequently, the alkyl radical undergoes a Giese-type reaction with the Dha derivative, forming a new C(sp3)–C(sp3) bond. The reaction can be performed in a phosphate-buffered saline (PBS) solution and shows post-functionalization prospects through pathways involving classical peptide chemistry.
Graphical Abstract
Introduction
The construction of C(sp3)–C(sp3) bonds is a highly important target in synthetic organic chemistry. Historically, polar conjugate additions have been a benchmark method for constructing these bonds by functionalizing an electron-deficient olefin [1-3]. Recently, however, radical-based approaches have also gained widespread attention for their unique advantages in these transformations [4]. Radical chemistry often exhibits complementary reactivity to two-electron pathways and can be performed with high selectivity, atom economy, and functional group tolerance [5]. A well-known radical pathway for the functionalization of an electron-deficient olefin is the Giese reaction (Figure 1) [6,7]. This reaction involves the hydroalkylation of the olefin via radical addition (RA), followed by either hydrogen-atom transfer (HAT) or single-electron transfer (SET) and protonation.
Traditionally, alkyl radicals have been produced from alkyl halides, using azobisisobutyronitrile (AIBN) as initiator, promoting a tin-mediated XAT (Figure 1a) [8,9]. However, tin-based compounds are highly toxic and require harsh conditions for the initiation event.
![[1860-5397-20-271-1]](/bjoc/content/figures/1860-5397-20-271-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Giese reaction: Radical addition on olefins with an electron-withdrawing group (EWG) followed by a HAT or SET and protonation; halogen-atom transfer: (a) tin-mediated XAT, (b) XAT initiated by a photocatalyst (PC) and mediated by boranes (B), silanes (Si) or alkylamines (N); state-of-the-art: (c) silane-mediated alkylation initiated by a photocatalyst, (d) silane-mediated alkylation initiated by photolysis of alkyl iodides, (e) silane-mediated alkylation initiated by photolysis of alkyl bromides in flow; this work: silane-mediated alkylation of Dha derivatives initiated by a photocatalyst.
Figure 1: Giese reaction: Radical addition on olefins with an electron-withdrawing group (EWG) followed by a ...
Fortunately, a renaissance in the field of photochemistry has introduced new ways of generating radicals like photoredox catalysis and via electron donor–acceptor (EDA) complexes [10-13]. These advances, coupled with modern electrochemical methods, chemical reactor engineering and light emitting diodes (LED), have eliminated the need for thermal radical activation, resulting in milder and safer reaction conditions [14-16]. Given the toxicity of tin-based compounds, there has been significant interest in developing alternative halogen-atom-transfer reagents. Borane, alkylamine, and silane compounds have emerged as effective XAT reagents upon photocatalytic activation (Figure 1b) [17-21]. A photocatalytic HAT or SET generates the corresponding boryl, α-amino or silyl radical, which can abstract a halogen atom from alkyl halides to form the corresponding alkyl radical.
However, the use of TTMS as a XAT reagent had already been established by Chatgilialoglu et al. [22] under non-photoredox conditions, MacMillan et al. [23] sparked renewed interest in silanes as XAT reagents by generating a tris(trimethylsilyl)silyl radical through photoredox catalysis for arylation reactions [22,23]. In 2018, Balsells et al. [24] reported a similar strategy to generate alkyl radicals and explored the feasibility of a Giese-type reaction (Figure 1c). More recently, Gaunt et al. [25] showed that irradiation of alkyl iodides combined with TTMS leads to the formation of an alkyl radical, which can be used in a Giese-type reaction without the need of a photocatalyst (Figure 1d) [25]. Noël et al. [26] have further extended this approach to include alkyl bromides (Figure 1e) [26]. Despite the effectiveness of the photolysis, benzophenone derivatives have also been shown to enhance the productivity of silane-mediated conjugate additions, using alkyl halides [27].
Amid the growing popularity of biomolecular drug candidates, the late-stage modification of peptide scaffolds has gained significant importance [28]. A particularly interesting class of amino acids for late-stage diversification consists of dehydroamino acids (Dha). Dha derivatives have shown modification potential by means of polar, metal-, or organo-catalyzed and radical additions [29-36]. As such, Dha derivatives make an excellent candidate for exploring a photochemical Giese-type reaction [37]. To foster a physiological reaction environment, reactions can be conducted in aqueous solution, meeting important requirements with regard to bioorthogonal chemistry [38].
Considering previous research that demonstrated photochemical hydrogen atom abstraction by benzophenone derivatives from trialkylsilyl hydrides [27], as well as advances in alkyl radical formation using these hydrides, we sought to combine these findings. Herein, we report a photochemical alkylation methodology targeting the olefin moiety of Dha derivatives, conducted in an aqueous solution for the aforementioned bioorthogonal advantages.
Results and Discussion
Inspired by previously conducted research concerning benzophenone hydrogen-atom transfer and silane-mediated activation of alkyl bromides to perform a photochemical Giese reaction, methyl 2-(1,3-dioxoisoindolin-2-yl)acrylate (1) and bromocyclohexane (2) were dissolved in CH3CN (0.1 M) together with a stoichiometric amount of tris(trimethylsilyl)silane and a substoichiometric amount of (4-methoxyphenyl)(4-(trifluoromethyl)phenyl)methanone (BP l) (Table 1) [26,27].
Table 1: Methyl 2-(1,3-dioxoisoindolin-2-yl)acrylate (1, 0.5 mmol), bromocyclohexane (2, 1.25 mmol), tris(trimethylsilyl)silane (0.55 mmol), (4-methoxyphenyl)(4-(trifluoromethyl)phenyl)methanone (0.1 mmol), PBS 0.2 M solution (2.5 mL), λ = 390 nm, 25 °C, overnight. The yield of 3 was calculated by 1H NMR with 1,1,2-trichloroethene as external standard.
|
|
|||
| Entry | Deviation from the reaction conditions | Conversion 1 (%) | NMR yield 3 (%) |
| 1 | CH3CN instead of PBS 0.2 M; 1.5 equiv (TMS)3SiH | 85 | 51 |
| 2 | CH3CN instead of PBS 0.2 M; no BP l; 1.5 equiv (TMS)3SiH | 90 | 38 |
| 3 | H2O instead of PBS 0.2 M; 1.5 equiv (TMS)3SiH | 100 | 58 |
| 4 | PBS 0.1 M instead of PBS 0.2 M | 100 | 44 |
| 5 | none | 100 | 60 |
| 6 | PBS 0.4 M instead of PBS 0.2 M | 100 | 60 |
| 7 | 3 hours | 100 | 67 |
The reaction was performed in batch, using a 390 nm Kessil UV-A lamp, stirring at 600 rpm overnight at 25 °C (see Supporting Information File 1 for a more detailed optimization).
The initial reaction, using CH3CN as solvent, led to formation of methyl 3-cyclohexyl-2-(1,3-dioxoisoindolin-2-yl)propanoate (3, 51% yield, 85% conv.; Table 1, entry 1). To demonstrate the importance of the photocatalyst, BP l was excluded (Table 1, entry 2), resulting in a slightly higher conversion and a decrease in product formation (38% yield, 90% conv.), meaning BP l increases the productivity of the reaction. Having established that the reaction works in CH3CN, we evaluated its compatibility with water. To our delight, the reaction in water provided full conversion and a higher yield than the one observed in CH3CN (58% yield, 100% conv.; Table 1, entry 3). These conditions were further refined by adding of phosphate-buffered saline and decreasing the amount of (TMS)3SiH to 1.1 equiv, as no further increase in yield was noticed during the optimization. Similar to the reaction in deionized water, all entries with PBS solution reached a full conversion. While the yield of the reaction with a 0.1 M PBS solution was slightly lower than that of the reaction in deionized water (44% yield, 100% conv.; Table 1, entry 4), a 0.2 M PBS solution resulted in an increased yield (60% yield, 100% conv.; Table 1, entry 5). Upon increasing the concentration of the PBS solution to 0.4 M, no change in yield was observed (60% yield, 100% conv.; Table 1, entry 6). Lastly, the optimal reaction time was determined to be 3 hours (67% yield, 100% conv.; Table 1, entry 7). Having optimized the reaction conditions, a scope of primary, secondary, and tertiary alkyl bromides and different Dha derivatives was investigated (Figure 2).
Figure 2: Alkyl bromide and Dha derivative scope. Reaction conditions: Dha derivative (0.5 mmol), alkyl bromide (1.25 mmol), tris(trimethylsilyl)silane (0.55 mmol), (4-methoxyphenyl)(4-(trifluoromethyl)phenyl)methanone (0.1 mmol), PBS 0.2 M solution (2.5 mL), λ = 390 nm, 25 °C. a4 hours of reaction time, b3 hours of reaction time. The isolated product yields are reported.
Figure 2: Alkyl bromide and Dha derivative scope. Reaction conditions: Dha derivative (0.5 mmol), alkyl bromi...
A slight modification of the reaction protocol was used in regard to primary and tertiary alkyl bromides, requiring a longer reaction time (4 hours instead of 3 hours) to achieve full conversion.
The reaction worked well with aliphatic primary bromides (4–10). However, a longer chain length slightly decreased the yield, comparing compound 4 (58%) to compound 5 (50%). The reaction was not influenced by the presence of phenyl rings, as the yield of compound 6 (60%) was comparable to the yield of 4. Besides, the introduction of a boronic pinacol ester group, useful for subsequent post-functionalization, compound 7 was achieved in a relatively lower yield of 36%. Interestingly, the reaction also worked adequately for primary alkyl bromides with electron-withdrawing groups as demonstrated in compounds 8 (46%) and 9 (47%). Moreover, primary alkyl bromides like bromomethylazetidines as used in compound 10 (53%) also resulted in decent yields. Acyclic secondary alkyl substrates all resulted in good yields (11–13, 50–62%). For the secondary alkyl substrates, a higher yield was notably obtained for compound 13 with a longer chain length than for 12. Similarly, the secondary cyclic alkyl substrates used in compound 18 (60%) and 3 (56%) worked well for this reaction. Besides, oxygen-containing compounds 14 and 15 were obtained in yields of 51% and 67% and medicinally interesting groups like the azetidine in compound 16 (56%) and the piperidine in compound 17 (67%) also resulted in good yields. Concerning tertiary alkyl substrates, acyclic alkyl substrates used in compound 19 (68%) and 20 (53%) as well as cyclic alkyl substrates like bromomethylcyclohexane used in compound 21 (61%) and bromoadamantane in compound 22 (58%) were obtained in satisfactory yields.
Subsequently, different Dha derivatives were subjected to the optimized reaction conditions, using bromocyclohexane (2) as the alkyl bromide. In order to explore the effect of the presence of an amide moiety in the Dha derivatives, a tertiary amide was firstly used to exclude selectivity issues arising from the hydrogen atoms of the amide functionality, yielding compound 23 in synthetically useful yield (39%). Interestingly, a secondary amide was formed in a higher yield of compound 24 (62%) compared to a Dha with a tertiary amide on the same position. Alternatively, the use of a double Boc-protected Dha resulted in a rather low yield of compound 25 (32%), while varying one Boc-protecting group with a Cbz-protecting group increased the yield substantially to 86% for compound 26. In addition, the use of a cyclic, N-Cbz-protected Michael acceptor, derived from proline, allowed for preparation of compound 27 with a 35% yield in 4 hours without control of diastereoselectivity (dr = 1:1).
Finally, to prove that the optimized reaction also works at a larger scale, the model reaction was carried out on a 2.2 mmol scale (Figure 3), obtaining a slightly elevated yield (67%) compared to compound 3, which was previously formed at a 0.5 mmol scale.
Figure 3: Scaled-up reaction. Reaction conditions: Dha derivative (2.2 mmol), alkyl bromide (5.4 mmol), tris(trimethylsilyl)silane (2.4 mmol), (4-methoxyphenyl)(4-(trifluoromethyl)phenyl)methanone (0.4 mmol), PBS 0.2 M solution (10.8 mL), λ = 390 nm, 25 °C, 4 hours, isolated product yield.
Figure 3: Scaled-up reaction. Reaction conditions: Dha derivative (2.2 mmol), alkyl bromide (5.4 mmol), tris(...
Conclusion
In conclusion, a photochemical methodology to promote the metal-free alkylation of dehydroalanine derivatives was developed, by means of silane-mediated alkyl bromide activation. The biocompatibility of the reaction enabled by the PBS solution and the mild photochemical reaction conditions makes the transformation useful for late-stage functionalization under physiological conditions. Besides, the reaction was successfully scaled up by roughly four times, leading to a slight increase in chemical yield.
Supporting Information
| Supporting Information File 1: 1H NMR, 13C NMR, and HRMS spectra of all the synthesized compounds. | ||
| Format: PDF | Size: 3.9 MB | Download |
Acknowledgements
We are grateful to University Research Services Center (CeSAR)-UniCA for the analysis service.
Funding
P. H. thanks the Erasmus+ traineeship program. The research contract (63-G-19602-1) of M.R. is co-financed by the European Union "FSE-REACT-EU, PON Research and innovation 2014-2020 DM 1062/2021" (CUP H31B21009610007). A.L. thanks the MUR for a Young Researchers Seal of Excellence fellowship (PlaDisPho) SOE_0000091 (PNRR).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Yang, L.; Huang, H. Chem. Rev. 2015, 115, 3468–3517. doi:10.1021/cr500610p
Return to citation in text: [1] -
Csákÿ, A. G.; de La Herrán, G.; Murcia, M. C. Chem. Soc. Rev. 2010, 39, 4080–4102. doi:10.1039/b924486g
Return to citation in text: [1] -
Ballini, R.; Bosica, G.; Fiorini, D.; Palmieri, A.; Petrini, M. Chem. Rev. 2005, 105, 933–972. doi:10.1021/cr040602r
Return to citation in text: [1] -
Renaud, P.; Sibi, M. P., Eds. Radicals in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2001. doi:10.1002/9783527618293
Return to citation in text: [1] -
Castellino, N. J.; Montgomery, A. P.; Danon, J. J.; Kassiou, M. Chem. Rev. 2023, 123, 8127–8153. doi:10.1021/acs.chemrev.2c00797
Return to citation in text: [1] -
Giese, B.; Meister, J. Angew. Chem., Int. Ed. Engl. 1977, 16, 178–179. doi:10.1002/anie.197701781
Return to citation in text: [1] -
Kitcatt, D. M.; Nicolle, S.; Lee, A.-L. Chem. Soc. Rev. 2022, 51, 1415–1453. doi:10.1039/d1cs01168e
Return to citation in text: [1] -
Kuivila, H. G.; Menapace, L. W. J. Org. Chem. 1963, 28, 2165–2167. doi:10.1021/jo01044a001
Return to citation in text: [1] -
Giese, B.; Dupuis, J. Angew. Chem., Int. Ed. Engl. 1983, 22, 622–623. doi:10.1002/anie.198306221
Return to citation in text: [1] -
Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898–6926. doi:10.1021/acs.joc.6b01449
Return to citation in text: [1] -
Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. J. Am. Chem. Soc. 2020, 142, 5461–5476. doi:10.1021/jacs.0c01416
Return to citation in text: [1] -
McClain, E. J.; Monos, T. M.; Mori, M.; Beatty, J. W.; Stephenson, C. R. J. ACS Catal. 2020, 10, 12636–12641. doi:10.1021/acscatal.0c03837
Return to citation in text: [1] -
Wortman, A. K.; Stephenson, C. R. J. Chem 2023, 9, 2390–2415. doi:10.1016/j.chempr.2023.06.013
Return to citation in text: [1] -
Yuan, Y.; Yang, J.; Lei, A. Chem. Soc. Rev. 2021, 50, 10058–10086. doi:10.1039/d1cs00150g
Return to citation in text: [1] -
Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S. D. A.; Noël, T. Chem. Rev. 2022, 122, 2752–2906. doi:10.1021/acs.chemrev.1c00332
Return to citation in text: [1] -
Wan, T.; Wen, Z.; Laudadio, G.; Capaldo, L.; Lammers, R.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Broersma, R.; Noël, T. ACS Cent. Sci. 2022, 8, 51–56. doi:10.1021/acscentsci.1c01109
Return to citation in text: [1] -
Chatgilialoglu, C.; Ferreri, C.; Landais, Y.; Timokhin, V. I. Chem. Rev. 2018, 118, 6516–6572. doi:10.1021/acs.chemrev.8b00109
Return to citation in text: [1] -
Zhang, Z.; Tilby, M. J.; Leonori, D. Nat. Synth. 2024, 3, 1221–1230. doi:10.1038/s44160-024-00587-5
Return to citation in text: [1] -
Constantin, T.; Zanini, M.; Regni, A.; Sheikh, N. S.; Juliá, F.; Leonori, D. Science 2020, 367, 1021–1026. doi:10.1126/science.aba2419
Return to citation in text: [1] -
Wan, T.; Capaldo, L.; Ravelli, D.; Vitullo, W.; de Zwart, F. J.; de Bruin, B.; Noël, T. J. Am. Chem. Soc. 2023, 145, 991–999. doi:10.1021/jacs.2c10444
Return to citation in text: [1] -
Poletti, L.; Massi, A.; Ragno, D.; Droghetti, F.; Natali, M.; De Risi, C.; Bortolini, O.; Di Carmine, G. Org. Lett. 2023, 25, 4862–4867. doi:10.1021/acs.orglett.3c01660
Return to citation in text: [1] -
Chatgilialoglu, C.; Griller, D.; Lesage, M. J. Org. Chem. 1988, 53, 3641–3642. doi:10.1021/jo00250a051
Return to citation in text: [1] [2] -
Zhang, P.; Le, C. C.; MacMillan, D. W. C. J. Am. Chem. Soc. 2016, 138, 8084–8087. doi:10.1021/jacs.6b04818
Return to citation in text: [1] [2] -
ElMarrouni, A.; Ritts, C. B.; Balsells, J. Chem. Sci. 2018, 9, 6639–6646. doi:10.1039/c8sc02253d
Return to citation in text: [1] -
Mistry, S.; Kumar, R.; Lister, A.; Gaunt, M. J. Chem. Sci. 2022, 13, 13241–13247. doi:10.1039/d2sc03516b
Return to citation in text: [1] [2] -
Fanini, F.; Luridiana, A.; Mazzarella, D.; Alfano, A. I.; van der Heide, P.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Noël, T. Tetrahedron Lett. 2023, 117, 154380. doi:10.1016/j.tetlet.2023.154380
Return to citation in text: [1] [2] [3] -
Luridiana, A.; Mazzarella, D.; Capaldo, L.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Jan Buma, W.; Noël, T. ACS Catal. 2022, 12, 11216–11225. doi:10.1021/acscatal.2c03805
Return to citation in text: [1] [2] [3] -
Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Signal Transduction Targeted Ther. 2022, 7, 48. doi:10.1038/s41392-022-00904-4
Return to citation in text: [1] -
Bogart, J. W.; Bowers, A. A. Org. Biomol. Chem. 2019, 17, 3653–3669. doi:10.1039/c8ob03155j
Return to citation in text: [1] -
Jousseaume, T.; Wurz, N. E.; Glorius, F. Angew. Chem., Int. Ed. 2011, 50, 1410–1414. doi:10.1002/anie.201006548
Return to citation in text: [1] -
Key, H. M.; Miller, S. J. J. Am. Chem. Soc. 2017, 139, 15460–15466. doi:10.1021/jacs.7b08775
Return to citation in text: [1] -
Retini, M.; Bartolucci, S.; Bartoccini, F.; Piersanti, G. Chem. – Eur. J. 2022, 28, e202201994. doi:10.1002/chem.202201994
Return to citation in text: [1] -
Kaplaneris, N.; Akdeniz, M.; Fillols, M.; Arrighi, F.; Raymenants, F.; Sanil, G.; Gryko, D. T.; Noël, T. Angew. Chem., Int. Ed. 2024, 63, e202403271. doi:10.1002/anie.202403271
Return to citation in text: [1] -
Aycock, R. A.; Pratt, C. J.; Jui, N. T. ACS Catal. 2018, 8, 9115–9119. doi:10.1021/acscatal.8b03031
Return to citation in text: [1] -
Sim, J.; Campbell, M. W.; Molander, G. A. ACS Catal. 2019, 9, 1558–1563. doi:10.1021/acscatal.8b04284
Return to citation in text: [1] -
Gu, X.; Zhang, Y.-A.; Zhang, S.; Wang, L.; Ye, X.; Occhialini, G.; Barbour, J.; Pentelute, B. L.; Wendlandt, A. E. Nature 2024, 634, 352–358. doi:10.1038/s41586-024-07988-8
Return to citation in text: [1] -
Peng, X.; Xu, K.; Zhang, Q.; Liu, L.; Tan, J. Trends Chem. 2022, 4, 643–657. doi:10.1016/j.trechm.2022.04.008
Return to citation in text: [1] -
Lim, R. K. V.; Lin, Q. Chem. Commun. 2010, 46, 1589. doi:10.1039/b925931g
Return to citation in text: [1]
| 29. | Bogart, J. W.; Bowers, A. A. Org. Biomol. Chem. 2019, 17, 3653–3669. doi:10.1039/c8ob03155j |
| 30. | Jousseaume, T.; Wurz, N. E.; Glorius, F. Angew. Chem., Int. Ed. 2011, 50, 1410–1414. doi:10.1002/anie.201006548 |
| 31. | Key, H. M.; Miller, S. J. J. Am. Chem. Soc. 2017, 139, 15460–15466. doi:10.1021/jacs.7b08775 |
| 32. | Retini, M.; Bartolucci, S.; Bartoccini, F.; Piersanti, G. Chem. – Eur. J. 2022, 28, e202201994. doi:10.1002/chem.202201994 |
| 33. | Kaplaneris, N.; Akdeniz, M.; Fillols, M.; Arrighi, F.; Raymenants, F.; Sanil, G.; Gryko, D. T.; Noël, T. Angew. Chem., Int. Ed. 2024, 63, e202403271. doi:10.1002/anie.202403271 |
| 34. | Aycock, R. A.; Pratt, C. J.; Jui, N. T. ACS Catal. 2018, 8, 9115–9119. doi:10.1021/acscatal.8b03031 |
| 35. | Sim, J.; Campbell, M. W.; Molander, G. A. ACS Catal. 2019, 9, 1558–1563. doi:10.1021/acscatal.8b04284 |
| 36. | Gu, X.; Zhang, Y.-A.; Zhang, S.; Wang, L.; Ye, X.; Occhialini, G.; Barbour, J.; Pentelute, B. L.; Wendlandt, A. E. Nature 2024, 634, 352–358. doi:10.1038/s41586-024-07988-8 |
| 27. | Luridiana, A.; Mazzarella, D.; Capaldo, L.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Jan Buma, W.; Noël, T. ACS Catal. 2022, 12, 11216–11225. doi:10.1021/acscatal.2c03805 |
| 28. | Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Signal Transduction Targeted Ther. 2022, 7, 48. doi:10.1038/s41392-022-00904-4 |
| 1. | Yang, L.; Huang, H. Chem. Rev. 2015, 115, 3468–3517. doi:10.1021/cr500610p |
| 2. | Csákÿ, A. G.; de La Herrán, G.; Murcia, M. C. Chem. Soc. Rev. 2010, 39, 4080–4102. doi:10.1039/b924486g |
| 3. | Ballini, R.; Bosica, G.; Fiorini, D.; Palmieri, A.; Petrini, M. Chem. Rev. 2005, 105, 933–972. doi:10.1021/cr040602r |
| 8. | Kuivila, H. G.; Menapace, L. W. J. Org. Chem. 1963, 28, 2165–2167. doi:10.1021/jo01044a001 |
| 9. | Giese, B.; Dupuis, J. Angew. Chem., Int. Ed. Engl. 1983, 22, 622–623. doi:10.1002/anie.198306221 |
| 26. | Fanini, F.; Luridiana, A.; Mazzarella, D.; Alfano, A. I.; van der Heide, P.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Noël, T. Tetrahedron Lett. 2023, 117, 154380. doi:10.1016/j.tetlet.2023.154380 |
| 6. | Giese, B.; Meister, J. Angew. Chem., Int. Ed. Engl. 1977, 16, 178–179. doi:10.1002/anie.197701781 |
| 7. | Kitcatt, D. M.; Nicolle, S.; Lee, A.-L. Chem. Soc. Rev. 2022, 51, 1415–1453. doi:10.1039/d1cs01168e |
| 26. | Fanini, F.; Luridiana, A.; Mazzarella, D.; Alfano, A. I.; van der Heide, P.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Noël, T. Tetrahedron Lett. 2023, 117, 154380. doi:10.1016/j.tetlet.2023.154380 |
| 5. | Castellino, N. J.; Montgomery, A. P.; Danon, J. J.; Kassiou, M. Chem. Rev. 2023, 123, 8127–8153. doi:10.1021/acs.chemrev.2c00797 |
| 25. | Mistry, S.; Kumar, R.; Lister, A.; Gaunt, M. J. Chem. Sci. 2022, 13, 13241–13247. doi:10.1039/d2sc03516b |
| 4. | Renaud, P.; Sibi, M. P., Eds. Radicals in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2001. doi:10.1002/9783527618293 |
| 25. | Mistry, S.; Kumar, R.; Lister, A.; Gaunt, M. J. Chem. Sci. 2022, 13, 13241–13247. doi:10.1039/d2sc03516b |
| 22. | Chatgilialoglu, C.; Griller, D.; Lesage, M. J. Org. Chem. 1988, 53, 3641–3642. doi:10.1021/jo00250a051 |
| 22. | Chatgilialoglu, C.; Griller, D.; Lesage, M. J. Org. Chem. 1988, 53, 3641–3642. doi:10.1021/jo00250a051 |
| 23. | Zhang, P.; Le, C. C.; MacMillan, D. W. C. J. Am. Chem. Soc. 2016, 138, 8084–8087. doi:10.1021/jacs.6b04818 |
| 27. | Luridiana, A.; Mazzarella, D.; Capaldo, L.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Jan Buma, W.; Noël, T. ACS Catal. 2022, 12, 11216–11225. doi:10.1021/acscatal.2c03805 |
| 17. | Chatgilialoglu, C.; Ferreri, C.; Landais, Y.; Timokhin, V. I. Chem. Rev. 2018, 118, 6516–6572. doi:10.1021/acs.chemrev.8b00109 |
| 18. | Zhang, Z.; Tilby, M. J.; Leonori, D. Nat. Synth. 2024, 3, 1221–1230. doi:10.1038/s44160-024-00587-5 |
| 19. | Constantin, T.; Zanini, M.; Regni, A.; Sheikh, N. S.; Juliá, F.; Leonori, D. Science 2020, 367, 1021–1026. doi:10.1126/science.aba2419 |
| 20. | Wan, T.; Capaldo, L.; Ravelli, D.; Vitullo, W.; de Zwart, F. J.; de Bruin, B.; Noël, T. J. Am. Chem. Soc. 2023, 145, 991–999. doi:10.1021/jacs.2c10444 |
| 21. | Poletti, L.; Massi, A.; Ragno, D.; Droghetti, F.; Natali, M.; De Risi, C.; Bortolini, O.; Di Carmine, G. Org. Lett. 2023, 25, 4862–4867. doi:10.1021/acs.orglett.3c01660 |
| 24. | ElMarrouni, A.; Ritts, C. B.; Balsells, J. Chem. Sci. 2018, 9, 6639–6646. doi:10.1039/c8sc02253d |
| 26. | Fanini, F.; Luridiana, A.; Mazzarella, D.; Alfano, A. I.; van der Heide, P.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Noël, T. Tetrahedron Lett. 2023, 117, 154380. doi:10.1016/j.tetlet.2023.154380 |
| 27. | Luridiana, A.; Mazzarella, D.; Capaldo, L.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Jan Buma, W.; Noël, T. ACS Catal. 2022, 12, 11216–11225. doi:10.1021/acscatal.2c03805 |
| 14. | Yuan, Y.; Yang, J.; Lei, A. Chem. Soc. Rev. 2021, 50, 10058–10086. doi:10.1039/d1cs00150g |
| 15. | Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S. D. A.; Noël, T. Chem. Rev. 2022, 122, 2752–2906. doi:10.1021/acs.chemrev.1c00332 |
| 16. | Wan, T.; Wen, Z.; Laudadio, G.; Capaldo, L.; Lammers, R.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Broersma, R.; Noël, T. ACS Cent. Sci. 2022, 8, 51–56. doi:10.1021/acscentsci.1c01109 |
| 37. | Peng, X.; Xu, K.; Zhang, Q.; Liu, L.; Tan, J. Trends Chem. 2022, 4, 643–657. doi:10.1016/j.trechm.2022.04.008 |
| 10. | Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898–6926. doi:10.1021/acs.joc.6b01449 |
| 11. | Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. J. Am. Chem. Soc. 2020, 142, 5461–5476. doi:10.1021/jacs.0c01416 |
| 12. | McClain, E. J.; Monos, T. M.; Mori, M.; Beatty, J. W.; Stephenson, C. R. J. ACS Catal. 2020, 10, 12636–12641. doi:10.1021/acscatal.0c03837 |
| 13. | Wortman, A. K.; Stephenson, C. R. J. Chem 2023, 9, 2390–2415. doi:10.1016/j.chempr.2023.06.013 |
| 23. | Zhang, P.; Le, C. C.; MacMillan, D. W. C. J. Am. Chem. Soc. 2016, 138, 8084–8087. doi:10.1021/jacs.6b04818 |
© 2024 van der Heide et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.