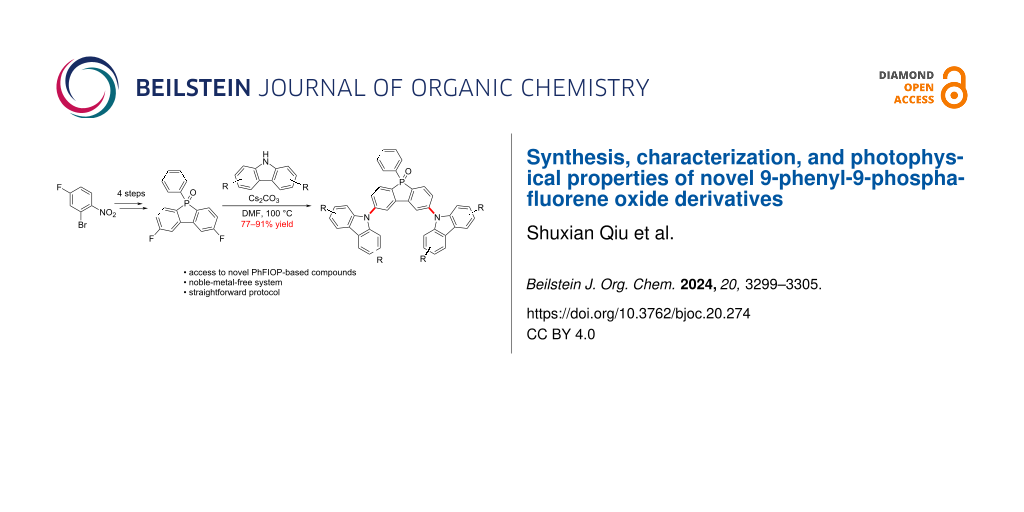
Abstract
A novel series of D−A−D-type 9-phenyl-9-phosphafluorene oxide (PhFlOP) derivatives was prepared and is reported herein. The synthetic protocol involved 5 steps from commercially available 2-bromo-4-fluoro-1-nitrobenzene, featuring a noble-metal-free system, mild reaction conditions, and a good yield, especially for the final Cs2CO3-facilitated nucleophilic substitution (77–91% yield). The characterization data obtained from IR and NMR spectroscopy (1H, 13C, 19F, and 31P) as well as HRMS spectrometry were in full agreement with the expected structures, and single-crystal X-ray diffraction analysis was conducted to confirm the structure of compound 7-H. Moreover, the photophysical properties of these PhFlOP derivatives were determined by UV–vis absorption and photoluminescence studies, revealing that their photophysical behavior can be affected by the different substituents in the donor carbazole group.
Graphical Abstract
Introduction
π-Conjugated molecular materials containing phosphine oxide (PO) groups have recently received considerable attention for their high thermal stability and unique optoelectronic features, and thus being widely applied in organic light-emitting diodes (OLEDs) [1,2]. To date, tremendous efforts have been devoted to the development of a variety of high-performing PO-based luminescent molecules [3-21] due to the benign electron injection/transport capability of PO-containing groups. Among them, 9-phenyl-9-phosphafluorene oxide (PhFlOP) is one of the most popular core units [22-26]. Compared to the traditional PO-containing moieties, PhFlOP possesses an enhanced rigid structure to reduce the possibility of nonradiative decay processes, which would improve optoelectronic properties [17,27].
Thermally activated delayed fluorescence (TADF) materials and devices have emerged rapidly in recent years, and they are mostly based on purely organic electron donor−electron acceptor (D−A) or D−A−D systems with significant intramolecular charge transfer interactions for frontier molecular orbital separation [28-30]. Due to the electron-accepting properties, PhFlOP can clearly act as an acceptor group in TADF emitters, indicating great potential for the development of highly efficient TADF molecules. In 2019, Nishida and co-workers prepared 5 D–A–D-type PhFlOP derivatives with electron-donating diarylamine or carbazole moieties in positions 2 and 8. They conducted optical and electrochemical studies, showing that the photophysical properties of PhFlOP depend on the nature of the electron-donating groups [31]. Later, Wu and co-workers introduced various electron donors to the PhFlOP unit to form new TADF emitters with high electroluminescence efficiency [32,33].
Despite this progress, TADF emitters containing the PhFlOP unit as an electron acceptor are still scarce. Meanwhile, the syntheses of the TADF emitters by the groups of Nishida and Wu both utilized palladium noble metal as a catalyst [31-33]. Therefore, it is of great significance to develop cost-effective synthetic access to PhFlOP-based TADF emitters. Additionally, the design of TADF emitters with the PhFlOP acceptor moiety and the carbazole donor moiety is lacking structural diversity. Herein, we present a 5-step synthesis of several novel D−A−D-type PhFlOP derivatives with substituted carbazole groups as donors, starting from commercially available 2-bromo-4-fluoro-1-nitrobenzene under noble-metal-free conditions. The structures and photophysical properties of the desired molecules were also determined.
Results and Discussion
Synthesis and structural characterization
The synthesis of the PhFlOP-based compounds 7 was achieved in 5 steps starting from commercially available 2-bromo-4-fluoro-1-nitrobenzene (1, Scheme 1 and Scheme 2). For the preparation of the key intermediate 5 (Scheme 1), self-coupling of 1 in the presence of copper followed by reduction of the nitro group generated diamine compound 3 (89% yield over 2 steps) [34]. Upon exposure to NaNO2/HCl, diamine 3 was transformed into a diazonium salt, which was captured by KI to deliver the diiodide 4. Treatment of 4 with n-BuLi, PhPCl2, and H2O2 sequentially gave 2,8-difluoro-5-phenylbenzo[b]phosphindole 5-oxide (5) in 68% yield.
Scheme 1: Preparation of key intermediate 5.
Scheme 1: Preparation of key intermediate 5.
Scheme 2: Synthesis of PhFlOP-based molecules 7.
Scheme 2: Synthesis of PhFlOP-based molecules 7.
With compound 5 in hand, we turned our attention to the synthesis of PhFlOP-based compounds through a Cs2CO3-facilitated nucleophilic substitution with substituted carbazoles as the nucleophiles (Scheme 2). For example, tert-butyl, bromo, carbazolyl, or phenyl substituents were introduced into the carbazoles. To our delight, by treatment of 5 with substituted carbazoles 6 in the presence of Cs2CO3 (5.0 equiv) in DMF at 100 °C, seven 2,8-bis(9H-carbazol-9-yl)-5-phenylbenzo[b]phosphindole 5-oxide derivatives 7 were furnished in good to excellent yields (77–91%). The structural characterization of the obtained molecules 2–7 was performed by NMR spectroscopy, which confirmed the synthetic outcomes (Figures S1–S11, Supporting Information File 1). The structures of compounds 7 were further confirmed by HRMS and IR analyses (Figures S12–S18, Supporting Information File 1).
In addition, the chemical structure of 7-H was fully elucidated by single-crystal X-ray crystallography, which was performed on a Bruker APEX-II CCD diffractometer using graphite monochromated Mo Kα radiation at a temperature of 296 ± 2 K. Crystallographic data were deposited with the Cambridge Crystallographic Data Centre under accession number CCDC 2256875. The crystallographic details are summarized in Table 1, and the structure of 7-H is shown in Figure 1 as an ORTEP diagram.
Table 1: Crystal data and structural parameters for 7-H.
| parameter | 7-H |
| empirical formula | C42H27N2OP |
| Fw | 606.19 |
| temperature (K) | 296(2) |
| crystal system | monoclinic |
| space group | P2(1)/c |
| a (Å) | 13.886(3) |
| b (Å) | 17.477(4) |
| c (Å) | 15.239(3) |
| α (deg) | 90 |
| β (deg) | 105.503(4) |
| γ (deg) | 90 |
| volume (Å3) | 3563.7(13) |
| Z | 4 |
| ρ calcd (mg/m3) | 1.586 |
| μ (Mo Kα, mm−1) | 0.612 |
| F(000) | 1702 |
| number of reflections | 26109 |
| unique reflections | 6289 |
| data/restraints/parameters | 8850/0/437 |
| Rint | 0.0253 |
| GOF (F2) | 1.062 |
| completeness to θ = 25.242 | 99.8% |
| final R indices [I > 2σ(I)] | R1 = 0.0769, wR2 = 0.2894 |
![[1860-5397-20-274-1]](/bjoc/content/figures/1860-5397-20-274-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: An ORTEP drawing obtained using the X-ray crystallographic data of 7-H.
Figure 1: An ORTEP drawing obtained using the X-ray crystallographic data of 7-H.
Photophysical properties
In order to investigate the photophysical properties of the PhFlOP-based molecules 7, UV−vis absorption and photoluminescence (PL) studies were conducted. UV−vis absorption spectra of 7 in toluene solution at room temperature are shown in Figure 2, and the corresponding data are included in Table 2. The spectra in Figure 2a exhibit two major absorption bands at ≈290 nm and ≈340 nm. The band at around 290 nm might be induced by π→π* transitions associated with the conjugated system, while the band at around 340 nm is attributed to intramolecular charge transfer processes. The low-energy absorption bands of 7-t-Bu (λmax = 345 nm, Table 2) and 7-Cz-2 (λmax = 342 nm) are slightly redshifted compared to 7-H (λmax = 338 nm), and larger redshifts are observed for 7-Ph-1 (λmax = 354 nm) and 7-Ph-2 (λmax = 366 nm). In contrast to 7-H, 7-Br (λmax = 327 nm) and 7-Cz-1 (λmax = 316 nm) show a blueshift. With a stronger electron-donating ability than 7-Cz-1, 7-Cz-2 shows a lower energy level for the absorption band stemming from intramolecular charge transfer, as indicated by the λmax value of 342 nm. In addition, the effect of solvent polarity on the UV−vis absorption was studied with 7-H (Figure 2b). The spectra show that there is no significant difference in the absorption bands in different solvents, indicating that the polar environment has insignificant effect on the molecular ground state of 7-H.
![[1860-5397-20-274-2]](/bjoc/content/figures/1860-5397-20-274-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: (a) UV–vis absorption spectra of the PhFlOP-based emitters 7 measured at a concentration of ≈10−5 M in toluene at room temperature. (b) UV–vis absorption spectra of 7-H measured at a concentration of ≈10−4 M in different solvents at room temperature.
Figure 2: (a) UV–vis absorption spectra of the PhFlOP-based emitters 7 measured at a concentration of ≈10−5 M...
Table 2: Photophysical data of the PhFlOP-based emitters 7.
| compound | λabs, nm (log ε)a | λem, nmb | PLQYc | τDF (ms)d |
| 7-H |
290 (4.52), 338 (4.21)
[291 (4.55), 338 (4.31)]e |
408 (412, 450, 478)f | 0.32 (0.16) | 1.94 (296) |
| 7-t-Bu | 295 (4.60), 345 (4.30) | 424 | 0.25 | 1.23 |
| 7-Br | 298 (4.70), 327 (4.29) | 383 | 0.22 | 0.88 |
| 7-Cz-1 | 285 (4.82), 316 (4.85) | 436 | 0.38 | 1.23 |
| 7-Cz-2 | 293 (4.84), 342 (4.41) | 444 | 0.31 | 1.15 |
| 7-Ph-1 | 285 (5.01), 354 (4.26) | 425 | 0.34 | 1.49 |
| 7-Ph-2 | 302 (4.68), 349 (4.56), 366 (4.63) | 392 | 0.27 | 1.17 |
aMeasured at a concentration of ≈10−5 M in toluene at room temperature. bMeasured in toluene at room temperature. cThe absolute PL quantum yield (PLQY) was measured in degassed toluene at room temperature using an integrating sphere, and the reported PLQY of solid 7-H is presented in parentheses [31]. dThe delayed fluorescence lifetime (τDF) was measured in degassed toluene at room temperature, and the reported τDF of 7-H in toluene at 77 K is presented in parentheses [31]. eReported data are presented in square brackets [31]. fThe values in parentheses are reported λem in various solvents, namely toluene, DCM, and CH3CN [31].
The PL spectra of the PhFlOP-based compounds 7 in toluene at room temperature are shown in Figure 3, and the λem values are included in Table 2. Different emission wavelengths are observed due to the various substituents present in the donor carbazole group (Figure 3a). Compared to 7-H (λem = 408 nm, Table 2), compounds 7-t-Bu (λem = 424 nm), 7-Cz-1 (λem = 436 nm), 7-Cz-2 (λem = 444 nm), and 7-Ph-1 (λem = 425 nm) all show a redshift due to the electron-donating groups (t-Bu, Cz, Ph) on the carbazole moiety. However, 7-Ph-2 exhibits a significantly blueshifted emission maximum at 392 nm, perhaps as a consequence of a more rigid configuration. As for 7-Br, owing to the electron-withdrawing properties of Br, it displays a blueshifted PL maximum at 383 nm. The emission wavelength of 7-Cz-2 has a slight redshift compared to 7-Cz-1, which may be induced by the stronger electron-donating feature of the carbazole substituent located on the donor carbazole group. In addition, we tested the emission wavelength of 7-H in different solvents (Figure 3b) and found that the maximum is redshifted gradually with increasing solvent polarity, which indicates the CT feature in the excited state. Further, the solvent dependence of 7-H exhibits good consistence with that reported by the Nishida group [31]. The PLQY and τDF values of the PhFlOP-based emitters 7 were measured in degassed toluene, and the corresponding data are included in Table 2, showing a PLQY ranging from 0.22–0.38 and a τDF in the order of milliseconds.
![[1860-5397-20-274-3]](/bjoc/content/figures/1860-5397-20-274-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) PL spectra of the PhFlOP-based emitters 7 measured in toluene at room temperature. (b) PL spectra of 7-H measured in different solvents at room temperature.
Figure 3: (a) PL spectra of the PhFlOP-based emitters 7 measured in toluene at room temperature. (b) PL spect...
Conclusion
In summary, we have developed a 5-step synthesis of a series of D−A−D-type PhFlOP derivatives 7 with 2-bromo-4-fluoro-1-nitrobenzene as the starting material. This novel protocol is mild, noble-metal-free, and operationally simple. The structure of 7-H was confirmed by single-crystal X-ray diffraction. Furthermore, UV–vis absorption and PL studies were carried out to explore the photophysical properties of these PhFlOP derivatives. Investigations for further applications of the PhFlOP-based emitters 7 are still ongoing.
Supporting Information
| Supporting Information File 1: General information, experimental procedures, characterization data, and copies of spectra. | ||
| Format: PDF | Size: 2.2 MB | Download |
Funding
This research was funded by the Youth Innovation Talents Project of Guangdong Universities (natural science) in China (No. 2022KQNCX052), Research and Innovation Team for Wastewater Treatment and Monitoring of Guangdong University of Education (No. 2024KYCXTD016), the quality and reform project of Guangdong province undergraduate teaching (No. XQSYS-2222873), and Guangdong Provincial Department of Education Key Area Special Project (2022ZDZX4037, 2024ZDZX2087, 2024ZDZX4063).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Song, X.; Xu, H. J. Inf. Disp. 2020, 21, 149–172. doi:10.1080/15980316.2020.1788657
Return to citation in text: [1] -
Yoshimura, A.; Misaki, Y. Chem. Rec. 2021, 21, 3520–3531. doi:10.1002/tcr.202100107
Return to citation in text: [1] -
Su, H.-C.; Fadhel, O.; Yang, C.-J.; Cho, T.-Y.; Fave, C.; Hissler, M.; Wu, C.-C.; Réau, R. J. Am. Chem. Soc. 2006, 128, 983–995. doi:10.1021/ja0567182
Return to citation in text: [1] -
Yamaguchi, E.; Wang, C.; Fukazawa, A.; Taki, M.; Sato, Y.; Sasaki, T.; Ueda, M.; Sasaki, N.; Higashiyama, T.; Yamaguchi, S. Angew. Chem., Int. Ed. 2015, 54, 4539–4543. doi:10.1002/anie.201500229
Return to citation in text: [1] -
Wang, B.; Lv, X.; Pan, B.; Tan, J.; Jin, J.; Wang, L. J. Mater. Chem. C 2015, 3, 11192–11201. doi:10.1039/c5tc02413g
Return to citation in text: [1] -
Lee, S. Y.; Adachi, C.; Yasuda, T. Adv. Mater. (Weinheim, Ger.) 2016, 28, 4626–4631. doi:10.1002/adma.201506391
Return to citation in text: [1] -
Duan, C.; Li, J.; Han, C.; Ding, D.; Yang, H.; Wei, Y.; Xu, H. Chem. Mater. 2016, 28, 5667–5679. doi:10.1021/acs.chemmater.6b01691
Return to citation in text: [1] -
Li, J.; Ding, D.; Wei, Y.; Zhang, J.; Xu, H. Adv. Opt. Mater. 2016, 4, 522–528. doi:10.1002/adom.201500673
Return to citation in text: [1] -
Yoshikai, N.; Santra, M.; Wu, B. Organometallics 2017, 36, 2637–2645. doi:10.1021/acs.organomet.7b00244
Return to citation in text: [1] -
Matsumura, M.; Yamada, M.; Muranaka, A.; Kanai, M.; Kakusawa, N.; Hashizume, D.; Uchiyama, M.; Yasuike, S. Beilstein J. Org. Chem. 2017, 13, 2304–2309. doi:10.3762/bjoc.13.226
Return to citation in text: [1] -
Guo, J.; Mao, C.; Deng, B.; Ye, L.; Yin, Y.; Gao, Y.; Tu, S. J. Org. Chem. 2020, 85, 6359–6371. doi:10.1021/acs.joc.0c00118
Return to citation in text: [1] -
Shen, Z.; Zhu, X.; Tang, W.; Feng, X. J.; Zhao, Z.; Lu, H. J. Mater. Chem. C 2020, 8, 9401–9409. doi:10.1039/d0tc01705a
Return to citation in text: [1] -
Ye, W.; Li, X.; Ding, B.; Wang, C.; Shrestha, M.; Ma, X.; Chen, Y.; Tian, H. J. Org. Chem. 2020, 85, 3879–3886. doi:10.1021/acs.joc.9b02847
Return to citation in text: [1] -
Duan, K.; Wang, D.; Yang, M.; Liu, Z.; Wang, C.; Tsuboi, T.; Deng, C.; Zhang, Q. ACS Appl. Mater. Interfaces 2020, 12, 30591–30599. doi:10.1021/acsami.0c02800
Return to citation in text: [1] -
Xu, S.; Huang, H.; Yuan, C.; Liu, F.; Ding, H.; Xiao, Q. Org. Chem. Front. 2021, 8, 1747–1755. doi:10.1039/d1qo00121c
Return to citation in text: [1] -
Haruna, B.; Hong, W.; Mohamed, W. I.; Guo, J.; Ye, L.; Yin, Y.; Gao, Y.; Tu, S. J. Org. Chem. 2021, 86, 13092–13099. doi:10.1021/acs.joc.1c00841
Return to citation in text: [1] -
Zhong, D.; Yu, Y.; Yue, L.; Yang, X.; Ma, L.; Zhou, G.; Wu, Z. Chem. Eng. J. 2021, 413, 127445. doi:10.1016/j.cej.2020.127445
Return to citation in text: [1] [2] -
Sk, B.; Thangaraji, V.; Yadav, N.; Nanda, G. P.; Das, S.; Gandeepan, P.; Zysman-Colman, E.; Rajamalli, P. J. Mater. Chem. C 2021, 9, 15583–15590. doi:10.1039/d1tc03849d
Return to citation in text: [1] -
Zhu, J.; Wei, D.; Wang, L.; Duan, Z. J. Org. Chem. 2022, 87, 11478–11490. doi:10.1021/acs.joc.2c01078
Return to citation in text: [1] -
Zhong, D.; Yang, X.; Deng, X.; Chen, X.; Sun, Y.; Tao, P.; Li, Z.; Zhang, J.; Zhou, G.; Wong, W.-Y. Chem. Eng. J. 2023, 452, 139480. doi:10.1016/j.cej.2022.139480
Return to citation in text: [1] -
Ledos, N.; Tondelier, D.; Geffroy, B.; Jacquemin, D.; Bouit, P.-A.; Hissler, M. J. Mater. Chem. C 2023, 11, 3826–3831. doi:10.1039/d3tc00245d
Return to citation in text: [1] -
Geramita, K.; McBee, J.; Tilley, T. D. J. Org. Chem. 2009, 74, 820–829. doi:10.1021/jo802171t
Return to citation in text: [1] -
Kabe, R.; Lynch, V. M.; Anzenbacher, P., Jr. CrystEngComm 2011, 13, 5423–5427. doi:10.1039/c1ce05388d
Return to citation in text: [1] -
Xu, X.; Guo, H.; Zhao, J.; Liu, B.; Yang, X.; Zhou, G.; Wu, Z. Chem. Mater. 2016, 28, 8556–8569. doi:10.1021/acs.chemmater.6b03177
Return to citation in text: [1] -
Mocanu, A.; Szűcs, R.; Caytan, E.; Roisnel, T.; Dorcet, V.; Bouit, P.-A.; Nyulászi, L.; Hissler, M. J. Org. Chem. 2019, 84, 957–962. doi:10.1021/acs.joc.8b02884
Return to citation in text: [1] -
Chi, X.; Luo, L.; Wu, L.; Ren, L.; Lin, J.; Zhang, Y.; Zeng, M.-H. J. Mol. Struct. 2021, 1226, 129401. doi:10.1016/j.molstruc.2020.129401
Return to citation in text: [1] -
Li, B.; Liu, M.; Sang, L.; Li, Z.; Wan, X.; Zhang, Y. Adv. Opt. Mater. 2023, 11, 2202610. doi:10.1002/adom.202202610
Return to citation in text: [1] -
Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M. P. Chem. Soc. Rev. 2017, 46, 915–1016. doi:10.1039/c6cs00368k
Return to citation in text: [1] -
Xie, F.-M.; Zhou, J.-X.; Li, Y.-Q.; Tang, J.-X. J. Mater. Chem. C 2020, 8, 9476–9494. doi:10.1039/d0tc02252g
Return to citation in text: [1] -
Tenopala-Carmona, F.; Lee, O. S.; Crovini, E.; Neferu, A. M.; Murawski, C.; Olivier, Y.; Zysman-Colman, E.; Gather, M. C. Adv. Mater. (Weinheim, Ger.) 2021, 33, 2100677. doi:10.1002/adma.202100677
Return to citation in text: [1] -
Nishida, J.-i.; Kawakami, Y.; Yamamoto, S.; Matsui, Y.; Ikeda, H.; Hirao, Y.; Kawase, T. Eur. J. Org. Chem. 2019, 3735–3743. doi:10.1002/ejoc.201900189
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Zhong, D.; Yu, Y.; Song, D.; Yang, X.; Zhang, Y.; Chen, X.; Zhou, G.; Wu, Z. ACS Appl. Mater. Interfaces 2019, 11, 27112–27124. doi:10.1021/acsami.9b05950
Return to citation in text: [1] [2] -
Chen, X.; Liu, S.; Sun, Y.; Zhong, D.; Feng, Z.; Yang, X.; Su, B.; Sun, Y.; Zhou, G.; Jiao, B.; Wu, Z. Mater. Chem. Front. 2023, 7, 1841–1854. doi:10.1039/d2qm01339h
Return to citation in text: [1] [2] -
Bhattacharjee, A.; Hosoya, H.; Ikeda, H.; Nishi, K.; Tsurugi, H.; Mashima, K. Chem. – Eur. J. 2018, 24, 11278–11282. doi:10.1002/chem.201801972
Return to citation in text: [1]
| 1. | Song, X.; Xu, H. J. Inf. Disp. 2020, 21, 149–172. doi:10.1080/15980316.2020.1788657 |
| 2. | Yoshimura, A.; Misaki, Y. Chem. Rec. 2021, 21, 3520–3531. doi:10.1002/tcr.202100107 |
| 28. | Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M. P. Chem. Soc. Rev. 2017, 46, 915–1016. doi:10.1039/c6cs00368k |
| 29. | Xie, F.-M.; Zhou, J.-X.; Li, Y.-Q.; Tang, J.-X. J. Mater. Chem. C 2020, 8, 9476–9494. doi:10.1039/d0tc02252g |
| 30. | Tenopala-Carmona, F.; Lee, O. S.; Crovini, E.; Neferu, A. M.; Murawski, C.; Olivier, Y.; Zysman-Colman, E.; Gather, M. C. Adv. Mater. (Weinheim, Ger.) 2021, 33, 2100677. doi:10.1002/adma.202100677 |
| 17. | Zhong, D.; Yu, Y.; Yue, L.; Yang, X.; Ma, L.; Zhou, G.; Wu, Z. Chem. Eng. J. 2021, 413, 127445. doi:10.1016/j.cej.2020.127445 |
| 27. | Li, B.; Liu, M.; Sang, L.; Li, Z.; Wan, X.; Zhang, Y. Adv. Opt. Mater. 2023, 11, 2202610. doi:10.1002/adom.202202610 |
| 22. | Geramita, K.; McBee, J.; Tilley, T. D. J. Org. Chem. 2009, 74, 820–829. doi:10.1021/jo802171t |
| 23. | Kabe, R.; Lynch, V. M.; Anzenbacher, P., Jr. CrystEngComm 2011, 13, 5423–5427. doi:10.1039/c1ce05388d |
| 24. | Xu, X.; Guo, H.; Zhao, J.; Liu, B.; Yang, X.; Zhou, G.; Wu, Z. Chem. Mater. 2016, 28, 8556–8569. doi:10.1021/acs.chemmater.6b03177 |
| 25. | Mocanu, A.; Szűcs, R.; Caytan, E.; Roisnel, T.; Dorcet, V.; Bouit, P.-A.; Nyulászi, L.; Hissler, M. J. Org. Chem. 2019, 84, 957–962. doi:10.1021/acs.joc.8b02884 |
| 26. | Chi, X.; Luo, L.; Wu, L.; Ren, L.; Lin, J.; Zhang, Y.; Zeng, M.-H. J. Mol. Struct. 2021, 1226, 129401. doi:10.1016/j.molstruc.2020.129401 |
| 31. | Nishida, J.-i.; Kawakami, Y.; Yamamoto, S.; Matsui, Y.; Ikeda, H.; Hirao, Y.; Kawase, T. Eur. J. Org. Chem. 2019, 3735–3743. doi:10.1002/ejoc.201900189 |
| 3. | Su, H.-C.; Fadhel, O.; Yang, C.-J.; Cho, T.-Y.; Fave, C.; Hissler, M.; Wu, C.-C.; Réau, R. J. Am. Chem. Soc. 2006, 128, 983–995. doi:10.1021/ja0567182 |
| 4. | Yamaguchi, E.; Wang, C.; Fukazawa, A.; Taki, M.; Sato, Y.; Sasaki, T.; Ueda, M.; Sasaki, N.; Higashiyama, T.; Yamaguchi, S. Angew. Chem., Int. Ed. 2015, 54, 4539–4543. doi:10.1002/anie.201500229 |
| 5. | Wang, B.; Lv, X.; Pan, B.; Tan, J.; Jin, J.; Wang, L. J. Mater. Chem. C 2015, 3, 11192–11201. doi:10.1039/c5tc02413g |
| 6. | Lee, S. Y.; Adachi, C.; Yasuda, T. Adv. Mater. (Weinheim, Ger.) 2016, 28, 4626–4631. doi:10.1002/adma.201506391 |
| 7. | Duan, C.; Li, J.; Han, C.; Ding, D.; Yang, H.; Wei, Y.; Xu, H. Chem. Mater. 2016, 28, 5667–5679. doi:10.1021/acs.chemmater.6b01691 |
| 8. | Li, J.; Ding, D.; Wei, Y.; Zhang, J.; Xu, H. Adv. Opt. Mater. 2016, 4, 522–528. doi:10.1002/adom.201500673 |
| 9. | Yoshikai, N.; Santra, M.; Wu, B. Organometallics 2017, 36, 2637–2645. doi:10.1021/acs.organomet.7b00244 |
| 10. | Matsumura, M.; Yamada, M.; Muranaka, A.; Kanai, M.; Kakusawa, N.; Hashizume, D.; Uchiyama, M.; Yasuike, S. Beilstein J. Org. Chem. 2017, 13, 2304–2309. doi:10.3762/bjoc.13.226 |
| 11. | Guo, J.; Mao, C.; Deng, B.; Ye, L.; Yin, Y.; Gao, Y.; Tu, S. J. Org. Chem. 2020, 85, 6359–6371. doi:10.1021/acs.joc.0c00118 |
| 12. | Shen, Z.; Zhu, X.; Tang, W.; Feng, X. J.; Zhao, Z.; Lu, H. J. Mater. Chem. C 2020, 8, 9401–9409. doi:10.1039/d0tc01705a |
| 13. | Ye, W.; Li, X.; Ding, B.; Wang, C.; Shrestha, M.; Ma, X.; Chen, Y.; Tian, H. J. Org. Chem. 2020, 85, 3879–3886. doi:10.1021/acs.joc.9b02847 |
| 14. | Duan, K.; Wang, D.; Yang, M.; Liu, Z.; Wang, C.; Tsuboi, T.; Deng, C.; Zhang, Q. ACS Appl. Mater. Interfaces 2020, 12, 30591–30599. doi:10.1021/acsami.0c02800 |
| 15. | Xu, S.; Huang, H.; Yuan, C.; Liu, F.; Ding, H.; Xiao, Q. Org. Chem. Front. 2021, 8, 1747–1755. doi:10.1039/d1qo00121c |
| 16. | Haruna, B.; Hong, W.; Mohamed, W. I.; Guo, J.; Ye, L.; Yin, Y.; Gao, Y.; Tu, S. J. Org. Chem. 2021, 86, 13092–13099. doi:10.1021/acs.joc.1c00841 |
| 17. | Zhong, D.; Yu, Y.; Yue, L.; Yang, X.; Ma, L.; Zhou, G.; Wu, Z. Chem. Eng. J. 2021, 413, 127445. doi:10.1016/j.cej.2020.127445 |
| 18. | Sk, B.; Thangaraji, V.; Yadav, N.; Nanda, G. P.; Das, S.; Gandeepan, P.; Zysman-Colman, E.; Rajamalli, P. J. Mater. Chem. C 2021, 9, 15583–15590. doi:10.1039/d1tc03849d |
| 19. | Zhu, J.; Wei, D.; Wang, L.; Duan, Z. J. Org. Chem. 2022, 87, 11478–11490. doi:10.1021/acs.joc.2c01078 |
| 20. | Zhong, D.; Yang, X.; Deng, X.; Chen, X.; Sun, Y.; Tao, P.; Li, Z.; Zhang, J.; Zhou, G.; Wong, W.-Y. Chem. Eng. J. 2023, 452, 139480. doi:10.1016/j.cej.2022.139480 |
| 21. | Ledos, N.; Tondelier, D.; Geffroy, B.; Jacquemin, D.; Bouit, P.-A.; Hissler, M. J. Mater. Chem. C 2023, 11, 3826–3831. doi:10.1039/d3tc00245d |
| 31. | Nishida, J.-i.; Kawakami, Y.; Yamamoto, S.; Matsui, Y.; Ikeda, H.; Hirao, Y.; Kawase, T. Eur. J. Org. Chem. 2019, 3735–3743. doi:10.1002/ejoc.201900189 |
| 34. | Bhattacharjee, A.; Hosoya, H.; Ikeda, H.; Nishi, K.; Tsurugi, H.; Mashima, K. Chem. – Eur. J. 2018, 24, 11278–11282. doi:10.1002/chem.201801972 |
| 31. | Nishida, J.-i.; Kawakami, Y.; Yamamoto, S.; Matsui, Y.; Ikeda, H.; Hirao, Y.; Kawase, T. Eur. J. Org. Chem. 2019, 3735–3743. doi:10.1002/ejoc.201900189 |
| 31. | Nishida, J.-i.; Kawakami, Y.; Yamamoto, S.; Matsui, Y.; Ikeda, H.; Hirao, Y.; Kawase, T. Eur. J. Org. Chem. 2019, 3735–3743. doi:10.1002/ejoc.201900189 |
| 32. | Zhong, D.; Yu, Y.; Song, D.; Yang, X.; Zhang, Y.; Chen, X.; Zhou, G.; Wu, Z. ACS Appl. Mater. Interfaces 2019, 11, 27112–27124. doi:10.1021/acsami.9b05950 |
| 33. | Chen, X.; Liu, S.; Sun, Y.; Zhong, D.; Feng, Z.; Yang, X.; Su, B.; Sun, Y.; Zhou, G.; Jiao, B.; Wu, Z. Mater. Chem. Front. 2023, 7, 1841–1854. doi:10.1039/d2qm01339h |
| 31. | Nishida, J.-i.; Kawakami, Y.; Yamamoto, S.; Matsui, Y.; Ikeda, H.; Hirao, Y.; Kawase, T. Eur. J. Org. Chem. 2019, 3735–3743. doi:10.1002/ejoc.201900189 |
| 32. | Zhong, D.; Yu, Y.; Song, D.; Yang, X.; Zhang, Y.; Chen, X.; Zhou, G.; Wu, Z. ACS Appl. Mater. Interfaces 2019, 11, 27112–27124. doi:10.1021/acsami.9b05950 |
| 33. | Chen, X.; Liu, S.; Sun, Y.; Zhong, D.; Feng, Z.; Yang, X.; Su, B.; Sun, Y.; Zhou, G.; Jiao, B.; Wu, Z. Mater. Chem. Front. 2023, 7, 1841–1854. doi:10.1039/d2qm01339h |
| 31. | Nishida, J.-i.; Kawakami, Y.; Yamamoto, S.; Matsui, Y.; Ikeda, H.; Hirao, Y.; Kawase, T. Eur. J. Org. Chem. 2019, 3735–3743. doi:10.1002/ejoc.201900189 |
| 31. | Nishida, J.-i.; Kawakami, Y.; Yamamoto, S.; Matsui, Y.; Ikeda, H.; Hirao, Y.; Kawase, T. Eur. J. Org. Chem. 2019, 3735–3743. doi:10.1002/ejoc.201900189 |
© 2024 Qiu et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.