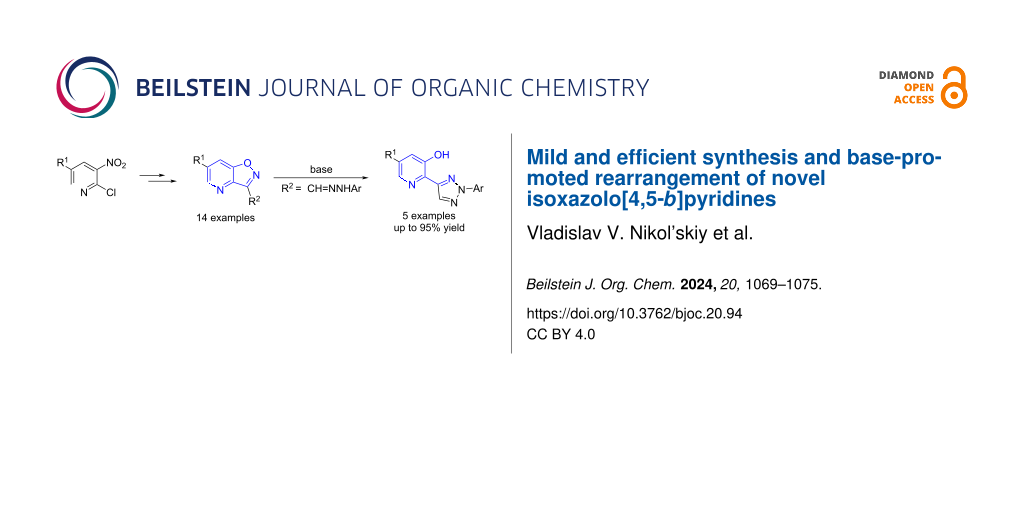
Abstract
An efficient method for the synthesis of isoxazolo[4,5-b]pyridines has been developed on the basis of readily available 2-chloro-3-nitropyridines via the intramolecular nucleophilic substitution of the nitro group as a key step. The previously unknown base-promoted Boulton–Katritzky rearrangement of isoxazolo[4,5-b]pyridine-3-carbaldehyde arylhydrazones into 3-hydroxy-2-(2-aryl[1,2,3]triazol-4-yl)pyridines was observed.
Graphical Abstract
Introduction
Nitrogen heterocycles represent a very important class of organic compounds that has found application in various fields of chemistry and materials science. These compounds are widespread in medicinal chemistry [1-3], production of high-energy-density compounds [4-7], and many others. In particular, isoxazolo[4,5-b]pyridines are of considerable interest due to their remarkable variety of biological activity, such as antibacterial [8], anticancer [9] or antiproliferative [10]. In addition, isoxazolo[4,5-b]pyridines were found to inhibit cytochrome P450 CYP17 responsible for the biosynthesis of androgens and estrogen precursors [11]. Some biologically active isoxazolo[4,5-b]pyridines are shown on Figure 1.
Figure 1: Some examples of biologically active isoxazolo[4,5-b]pyridines with antibacterial [8], anticancer [12] and cytotoxic [10,13] acitivities.
Figure 1: Some examples of biologically active isoxazolo[4,5-b]pyridines with antibacterial [8], anticancer [12] and...
A number of isoxazolo[4,5-b]pyridines has been described in patents, however, there are only a few methods for their synthesis reported in the literature. First representatives of this heterocyclic system were described by Gewald et al. in 1980 [14]. The known methods are usually based on either annulation of an isoxazole fragment to a pyridine cycle or vice versa formation of a pyridine ring based on appropriately substituted isoxazoles. In the first case (Scheme 1A) 3-halo- [11,15-19] or 3-hydroxypyridines [8,20] bearing a suitable functionality in position 2 were used for the cyclization. Alternatively (Scheme 1B), isoxazolo[4,5-b]pyridines can be constructed via intramolecular cyclization of 4-(propargylamino)isoxazoles [21] or through reactions of 4-amino-5-benzoylisoxazoles with ketones or 1,3-dicarbonyl compounds [10,13]. These and some additional examples of isoxazolo[4,5-b]pyridine core synthesis have been summarized in a microreview [22].
Scheme 1: Methods for the synthesis of isoxazolo[4,5-b]pyridines: (A) annulation of an isoxazole fragment to a pyridine ring; (B) annulation of a pyridine ring to a functionalized isoxazole core; (C) synthesis from available 3-nitropyridines.
Scheme 1: Methods for the synthesis of isoxazolo[4,5-b]pyridines: (A) annulation of an isoxazole fragment to ...
Here, we wish to report an efficient method for the synthesis of isoxazolo[4,5-b]pyridines bearing electron-withdrawing groups (EWG) at positions 3 and 6 starting from readily available 2-chloro-3-nitro-6-R-pyridines as shown in Scheme 1C. Since the key step of the synthesis is the intramolecular nucleophilic substitution of the aromatic nitro group, we assumed that the presence of an electron-withdrawing substituent at the pyridine ring would facilitate this transformation.
Results and Discussion
According to the general synthetic scheme (Scheme 1C), commercially available 2-chloro-3-nitropyridines 1a–c were reacted with ethyl acetoacetate in the presence of NaH to give compounds 2a–c which were not isolated and directly subjected to an in situ nitrosation affording isonitroso compounds 3a–c in good yields. Cyclization of the latter under the action of K2CO3 in MeCN at room temperature gave previously unknown ethyl isoxazolo[4,5-b]pyridine-3-carboxylates 4a–c (Scheme 2).
Scheme 2: Synthesis of ethyl 6-R-isoxazolo[4,5-b]pyridine-3-carboxylates 4a–c.
Scheme 2: Synthesis of ethyl 6-R-isoxazolo[4,5-b]pyridine-3-carboxylates 4a–c.
To the best of our knowledge only one compound (ethyl 5,7-dimethylisoxazolo[4,5-b]pyridine-3-carboxylate) has been synthesized using a similar method, however, the cyclization occurred under drastic conditions (NaH, DMF, 130 °C) as it was reported in patent literature [23].
We assumed that a similar synthetic route (nitrosation/SNAr) would be applicable for the synthesis of isoxazolo[4,5-b]pyridine derivatives bearing other EWG in position 3, for example a formyl group. Thus, the key isonitroso compounds 7 were synthesized from chlorides 1a–c via in situ formation of pyridylacetoacetic esters 2a–c followed by decarbonylation to give 2-methyl-3-nitropyridines 5a–c [24] which were used in the next step without purification. Their reactions with DMF-DMA afforded enamines 6 which, upon nitrosation, were converted into oximes 7a–c in moderate yields (Scheme 3).
Scheme 3: Synthesis of isonitroso compounds 7.
Scheme 3: Synthesis of isonitroso compounds 7.
When compounds 7 were treated with K2CO3 3-hydroxypyridine-2-carbonitriles 8 were obtained as sole products (Scheme 4). Apparently, a cyclization of oximes 7 to 3-formylisoxazolo[4,5-b]pyridine took place, followed by a base-promoted decarbonylation/isoxazole ring opening. Such transformations have been previously reported for benzo[d]isoxazoles with a carbonyl or carboxyl group in position 3 or 3-unsubstituted benzo[d]isoxazoles [25-29]. This means that the formyl group of compounds 7 should be protected prior to the attempted isoxazole ring formation. Indeed, reactions of 7a–c with ethylene glycol gave dioxolane derivatives 9a–c which were converted into isoxazolo[4,5-b]pyridines 10a–c in high yields under mild conditions (Scheme 4).
Scheme 4: Base-promoted cyclization of compounds 7a–c.
Scheme 4: Base-promoted cyclization of compounds 7a–c.
The obtained result prompted us to try another protecting group for the formyl function, namely arylhydrazone. Thus, reactions of compounds 7a–c with a number of arylhydrazines afforded the corresponding hydrazones 11 which were not isolated and subjected to cyclization under the action of K2CO3 (Scheme 5). In most cases the isoxazolo[4,5-b]pyridines 12 were obtained in pure form, however, cyclization of hydrazone 11a provided an inseparable mixture of two compounds which could be attributed to the target isoxazolo[4,5-b]pyridine 12a and triazole 13a formed as a result of Boulton–Katritzky rearrangement (Scheme 5). When this mixture was treated with K2CO3 in DMF at 60 °C, compound 13a was isolated in 92% yield (from 7a) (Table 1, entry 1). Such rearrangement has been reported previously for the benzo[d]isoxazole series [30], however, it has not been observed for isoxazolo[4,5-b]pyridine derivatives. It was found that the similar rearrangement of the other arylhydrazones 12b–h strongly depends on the aryl substituent. Indeed, the 2,4-dinitrophenylhydrazones 12b,e, and h did not undergo recyclization even under drastic conditions, apparently due to a low nucleophilicity of the hydrazone anion (Table 1, entries 2, 5, and 8). All other compounds 12 bearing no electron-withdrawing groups in the aryl moiety readily afforded the corresponding triazole derivatives in high yields under relatively mild conditions (K2CO3, DMF, 60 °C, Scheme 5). Substituents in the pyridine ring did not affect this transformation thus indicating that they do not participate in the stabilization of the pyridine-3-olate anion.
Scheme 5: Synthesis and rearrangement of arylhydrazones 12.
Scheme 5: Synthesis and rearrangement of arylhydrazones 12.
Table 1: Yields of compounds 12 and 13.
| Entry | R | Ar | Compound 12, yield (%) | Compound 13, yield (%) |
| 1 | NO2 | C6H5 | 12a, not isolated | 13a, 92 |
| 2 | NO2 | 2,4-(NO2)2C6H3 | 12b, 87 | 13b, n.r.a |
| 3 | CF3 | C6H5 | 12c, 85 | 13c, 95 |
| 4 | CF3 | 2-Cl-C6H4 | 12d, 82 | 13d, 91 |
| 5 | CF3 | 2,4-(NO2)2C6H3 | 12e, 71 | 13e, n.r. |
| 6 | Cl | C6H5 | 12f, 79 | 13f, 90 |
| 7 | Cl | 4-CH3-C6H4 | 12g, 76 | 13g, 95 |
| 8 | Cl | 2,4-(NO2)2C6H3 | 12h, 74 | 13h, n.r. |
aNo reaction.
It should be noted that the 4-(2-pyridyl)[1,2,3]triazole fragment is part of some pharmaceutically oriented molecules such as tradipitant, an experimental neurokinin-1 receptor antagonist [31], MU1787, a highly selective inhibitor of homeodomain-interacting protein kinases (HIPKs) [32], and combretastatin A-4 analogs evaluated for their anticancer properties against a panel of 60 human cancer cell lines [33] (Figure 2).
Figure 2: Biologically active analogs of compounds 13.
Figure 2: Biologically active analogs of compounds 13.
The structures of all new compounds were confirmed by 1H and 13C NMR and HRMS. X-ray diffraction studies were performed for compounds 12c and 13c,d (Figure 3; see Supporting Information File 1 for details) that allowed us to unambiguously establish the structures of both the starting hydrazones and rearrangement products.
![[1860-5397-20-94-3]](/bjoc/content/figures/1860-5397-20-94-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: X-ray crystal structures of compounds 12c (top left; the second crystallographically unique molecule is not shown), 13c (top right), and 13d (bottom) with thermal ellipsoids set at a 50% probability level. Intermolecular hydrogen bonds are drawn with dashed lines.
Figure 3: X-ray crystal structures of compounds 12c (top left; the second crystallographically unique molecul...
Conclusion
In summary, we have developed an efficient method for the synthesis of isoxazolo[4,5-b]pyridines based on the intramolecular nucleophilic substitution of the nitro group. The method comprises readily available starting materials, mild reaction conditions, easy work-up and high product yields. It was found that isoxazolo[4,5-b]pyridine-3-carbaldehyde arylhydrazones readily undergo a base-promoted Boulton–Katritzky rearrangement to give otherwise inaccessible 3-hydroxy-2-(2-aryl[1,2,3]triazol-4-yl)pyridines in excellent yields. As a result, a wide range of polyfunctional pyridines was synthesized, which can be considered as prospective platform for the design of pharmacology-oriented heterocyclic systems.
Supporting Information
| Supporting Information File 1: Experimental section, NMR spectra and X-ray analysis data. | ||
| Format: PDF | Size: 6.2 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Heravi, M. M.; Zadsirjan, V. RSC Adv. 2020, 10, 44247–44311. doi:10.1039/d0ra09198g
Return to citation in text: [1] -
Kumar, A.; Singh, A. K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J. P.; Pathak, P.; Grishina, M.; Verma, A.; Khalilullah, H.; Jaremko, M.; Emwas, A.-H.; Kumar, P. Pharmaceuticals 2023, 16, 299. doi:10.3390/ph16020299
Return to citation in text: [1] -
Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K. K.; Jonnalagadda, S. B. Molecules 2020, 25, 1909. doi:10.3390/molecules25081909
Return to citation in text: [1] -
Tang, J.; Yang, H.-w.; Cheng, G.-b. Energ. Mater. Front. 2023, 4, 110–122. doi:10.1016/j.enmf.2023.05.003
Return to citation in text: [1] -
Yin, P.; Shreeve, J. M. Adv. Heterocycl. Chem. 2017, 121, 89–131. doi:10.1016/bs.aihch.2016.04.004
Return to citation in text: [1] -
Makhova, N. N.; Belen’kii, L. I.; Gazieva, G. A.; Dalinger, I. L.; Konstantinova, L. S.; Kuznetsov, V. V.; Kravchenko, A. N.; Krayushkin, M. M.; Rakitin, O. A.; Starosotnikov, A. M.; Fershtat, L. L.; Shevelev, S. A.; Shirinian, V. Z.; Yarovenko, V. N. Russ. Chem. Rev. 2020, 89, 55–124. doi:10.1070/rcr4914
Return to citation in text: [1] -
Zlotin, S. G.; Dalinger, I. L.; Makhova, N. N.; Tartakovsky, V. A. Russ. Chem. Rev. 2020, 89, 1–54. doi:10.1070/rcr4908
Return to citation in text: [1] -
Thalji, R. K.; Raha, K.; Andreotti, D.; Checchia, A.; Cui, H.; Meneghelli, G.; Profeta, R.; Tonelli, F.; Tommasi, S.; Bakshi, T.; Donovan, B. T.; Howells, A.; Jain, S.; Nixon, C.; Quinque, G.; McCloskey, L.; Bax, B. D.; Neu, M.; Chan, P. F.; Stavenger, R. A. Bioorg. Med. Chem. Lett. 2019, 29, 1407–1412. doi:10.1016/j.bmcl.2019.03.029
Return to citation in text: [1] [2] [3] -
Rajanarendar, E.; Raju, S.; Reddy, M. N.; Krishna, S. R.; Kiran, L. H.; Narasimha Reddy, A. R.; Reddy, Y. N. Eur. J. Med. Chem. 2012, 50, 274–279. doi:10.1016/j.ejmech.2012.02.004
Return to citation in text: [1] -
Poręba, K.; Wietrzyk, J. Adv. Clin. Exp. Med. 2012, 21, 563–571.
Return to citation in text: [1] [2] [3] -
Balog, J. A.; Huang, A.; Velaparthi, U.; Liu, P. Substituted bicyclic heteroaryl compounds. WO Patent WO2013049263, April 4, 2013.
Return to citation in text: [1] [2] -
Rajanarendar, E.; Govardhan Reddy, K.; Ramakrishna, S.; Nagi Reddy, M.; Shireesha, B.; Durgaiah, G.; Reddy, Y. N. Bioorg. Med. Chem. Lett. 2012, 22, 6677–6680. doi:10.1016/j.bmcl.2012.08.098
Return to citation in text: [1] -
Poręba, K.; Wietrzyk, J. Isoxazolo[4,5-b]pyridine derivative of the chemical name amide of 6-benzoyl-5,7-diphenyloisoxazolo[4,5-b]pyridine-3-carboxylic acid and antiproliferative activity and process for the preparation thereof. Pol. Patent 215605, Dec 31, 2013.
Return to citation in text: [1] [2] -
Gewald, K.; Bellmann, P.; Jänsch, H.-J. Liebigs Ann. Chem. 1980, 1623–1629. doi:10.1002/jlac.198019801018
Return to citation in text: [1] -
Engers, D. W.; Blobaum, A. L.; Gogliotti, R. D.; Cheung, Y.-Y.; Salovich, J. M.; Garcia-Barrantes, P. M.; Daniels, J. S.; Morrison, R.; Jones, C. K.; Soars, M. G.; Zhuo, X.; Hurley, J.; Macor, J. E.; Bronson, J. J.; Conn, P. J.; Lindsley, C. W.; Niswender, C. M.; Hopkins, C. R. ACS Chem. Neurosci. 2016, 7, 1192–1200. doi:10.1021/acschemneuro.6b00035
Return to citation in text: [1] -
Conn, P. J.; Hopkins, C. R.; Lindsley, C. W.; Niswender, C. M.; Engers, D. W.; Panarese, J.; Bollinger, S.; Engers, J. Benzoisoxazole-substituted compounds as MGLUR4 allosteric potentiators, compositions, and methods of treating neurological dysfunction. WO Patent WO2016115282, July 21, 2016.
Return to citation in text: [1] -
Yu, W.; Bulger, P. G.; Maloney, K. M. Green Chem. 2016, 18, 4941–4946. doi:10.1039/c6gc01125j
Return to citation in text: [1] -
Tichenor, M. S.; Keith, J. M.; Jones, W. M.; Pierce, J. M.; Merit, J.; Hawryluk, N.; Seierstad, M.; Palmer, J. A.; Webb, M.; Karbarz, M. J.; Wilson, S. J.; Wennerholm, M. L.; Woestenborghs, F.; Beerens, D.; Luo, L.; Brown, S. M.; De Boeck, M.; Chaplan, S. R.; Breitenbucher, J. G. Bioorg. Med. Chem. Lett. 2012, 22, 7357–7362. doi:10.1016/j.bmcl.2012.10.076
Return to citation in text: [1] -
Hong, Y. R.; Kim, H. T.; Ro, S.; Cho, J. M.; Lee, S. H.; Kim, I. S.; Jung, Y. H. Bioorg. Med. Chem. Lett. 2014, 24, 3142–3145. doi:10.1016/j.bmcl.2014.05.003
Return to citation in text: [1] -
Hufnagel, B.; Zhu, W. F.; Franz, H. M.; Proschak, E.; Hernandez-Olmos, V. ChemistryOpen 2022, 11, e202200252. doi:10.1002/open.202200252
Return to citation in text: [1] -
Morita, T.; Fukuhara, S.; Fuse, S.; Nakamura, H. Org. Lett. 2018, 20, 433–436. doi:10.1021/acs.orglett.7b03760
Return to citation in text: [1] -
Nikol’skiy, V. V.; Starosotnikov, A. M. Chem. Heterocycl. Compd. 2023, 59, 240–242. doi:10.1007/s10593-023-03186-y
Return to citation in text: [1] -
Skerlj, R. T.; Bourque, E. M. J.; Greenlee, W. J.; Lansbury, P. T. Substituted imidazo[1,2-b]pyridazines, substituted imidazo[1,5-b]pyridazines, related compounds, and their use in the treatment of medical disorders. WO Patent WO2017192930, Nov 9, 2017.
Return to citation in text: [1] -
Nikol’skiy, V. V.; Minyaev, M. E.; Bastrakov, M. A.; Starosotnikov, A. M. Molecules 2022, 27, 5692. doi:10.3390/molecules27175692
Return to citation in text: [1] -
Vinogradov, V. M.; Dalinger, I. L.; Starosotnikov, A. M.; Shevelev, S. A. Mendeleev Commun. 2000, 10, 140–141. doi:10.1070/mc2000v010n04abeh001322
Return to citation in text: [1] -
Vinogradov, V. M.; Dalinger, I. L.; Starosotnikov, A. M.; Shevelev, S. A. Russ. Chem. Bull. 2001, 50, 464–469. doi:10.1023/a:1011313324497
Return to citation in text: [1] -
Meyer, V. Ber. Dtsch. Chem. Ges. 1893, 26, 1250–1257. doi:10.1002/cber.18930260216
Return to citation in text: [1] -
Brady, O. L.; Bishop, G. J. Chem. Soc., Trans. 1925, 127, 1357–1362. doi:10.1039/ct9252701357
Return to citation in text: [1] -
Borsche, W. Justus Liebigs Ann. Chem. 1912, 390, 1–29. doi:10.1002/jlac.19123900102
Return to citation in text: [1] -
Starosotnikov, A. M.; Vinogradov, V. M.; Kachala, V. V.; Shevelev, S. A. Russ. Chem. Bull. 2002, 51, 1519–1522. doi:10.1023/a:1020927410104
Return to citation in text: [1] -
George, D. T.; Gilman, J.; Hersh, J.; Thorsell, A.; Herion, D.; Geyer, C.; Peng, X.; Kielbasa, W.; Rawlings, R.; Brandt, J. E.; Gehlert, D. R.; Tauscher, J. T.; Hunt, S. P.; Hommer, D.; Heilig, M. Science 2008, 319, 1536–1539. doi:10.1126/science.1153813
Return to citation in text: [1] -
Němec, V.; Maier, L.; Berger, B.-T.; Chaikuad, A.; Drápela, S.; Souček, K.; Knapp, S.; Paruch, K. Eur. J. Med. Chem. 2021, 215, 113299. doi:10.1016/j.ejmech.2021.113299
Return to citation in text: [1] -
Madadi, N. R.; Penthala, N. R.; Howk, K.; Ketkar, A.; Eoff, R. L.; Borrelli, M. J.; Crooks, P. A. Eur. J. Med. Chem. 2015, 103, 123–132. doi:10.1016/j.ejmech.2015.08.041
Return to citation in text: [1]
| 30. | Starosotnikov, A. M.; Vinogradov, V. M.; Kachala, V. V.; Shevelev, S. A. Russ. Chem. Bull. 2002, 51, 1519–1522. doi:10.1023/a:1020927410104 |
| 24. | Nikol’skiy, V. V.; Minyaev, M. E.; Bastrakov, M. A.; Starosotnikov, A. M. Molecules 2022, 27, 5692. doi:10.3390/molecules27175692 |
| 25. | Vinogradov, V. M.; Dalinger, I. L.; Starosotnikov, A. M.; Shevelev, S. A. Mendeleev Commun. 2000, 10, 140–141. doi:10.1070/mc2000v010n04abeh001322 |
| 26. | Vinogradov, V. M.; Dalinger, I. L.; Starosotnikov, A. M.; Shevelev, S. A. Russ. Chem. Bull. 2001, 50, 464–469. doi:10.1023/a:1011313324497 |
| 27. | Meyer, V. Ber. Dtsch. Chem. Ges. 1893, 26, 1250–1257. doi:10.1002/cber.18930260216 |
| 28. | Brady, O. L.; Bishop, G. J. Chem. Soc., Trans. 1925, 127, 1357–1362. doi:10.1039/ct9252701357 |
| 29. | Borsche, W. Justus Liebigs Ann. Chem. 1912, 390, 1–29. doi:10.1002/jlac.19123900102 |
| 1. | Heravi, M. M.; Zadsirjan, V. RSC Adv. 2020, 10, 44247–44311. doi:10.1039/d0ra09198g |
| 2. | Kumar, A.; Singh, A. K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J. P.; Pathak, P.; Grishina, M.; Verma, A.; Khalilullah, H.; Jaremko, M.; Emwas, A.-H.; Kumar, P. Pharmaceuticals 2023, 16, 299. doi:10.3390/ph16020299 |
| 3. | Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K. K.; Jonnalagadda, S. B. Molecules 2020, 25, 1909. doi:10.3390/molecules25081909 |
| 22. | Nikol’skiy, V. V.; Starosotnikov, A. M. Chem. Heterocycl. Compd. 2023, 59, 240–242. doi:10.1007/s10593-023-03186-y |
| 9. | Rajanarendar, E.; Raju, S.; Reddy, M. N.; Krishna, S. R.; Kiran, L. H.; Narasimha Reddy, A. R.; Reddy, Y. N. Eur. J. Med. Chem. 2012, 50, 274–279. doi:10.1016/j.ejmech.2012.02.004 |
| 23. | Skerlj, R. T.; Bourque, E. M. J.; Greenlee, W. J.; Lansbury, P. T. Substituted imidazo[1,2-b]pyridazines, substituted imidazo[1,5-b]pyridazines, related compounds, and their use in the treatment of medical disorders. WO Patent WO2017192930, Nov 9, 2017. |
| 8. | Thalji, R. K.; Raha, K.; Andreotti, D.; Checchia, A.; Cui, H.; Meneghelli, G.; Profeta, R.; Tonelli, F.; Tommasi, S.; Bakshi, T.; Donovan, B. T.; Howells, A.; Jain, S.; Nixon, C.; Quinque, G.; McCloskey, L.; Bax, B. D.; Neu, M.; Chan, P. F.; Stavenger, R. A. Bioorg. Med. Chem. Lett. 2019, 29, 1407–1412. doi:10.1016/j.bmcl.2019.03.029 |
| 21. | Morita, T.; Fukuhara, S.; Fuse, S.; Nakamura, H. Org. Lett. 2018, 20, 433–436. doi:10.1021/acs.orglett.7b03760 |
| 4. | Tang, J.; Yang, H.-w.; Cheng, G.-b. Energ. Mater. Front. 2023, 4, 110–122. doi:10.1016/j.enmf.2023.05.003 |
| 5. | Yin, P.; Shreeve, J. M. Adv. Heterocycl. Chem. 2017, 121, 89–131. doi:10.1016/bs.aihch.2016.04.004 |
| 6. | Makhova, N. N.; Belen’kii, L. I.; Gazieva, G. A.; Dalinger, I. L.; Konstantinova, L. S.; Kuznetsov, V. V.; Kravchenko, A. N.; Krayushkin, M. M.; Rakitin, O. A.; Starosotnikov, A. M.; Fershtat, L. L.; Shevelev, S. A.; Shirinian, V. Z.; Yarovenko, V. N. Russ. Chem. Rev. 2020, 89, 55–124. doi:10.1070/rcr4914 |
| 7. | Zlotin, S. G.; Dalinger, I. L.; Makhova, N. N.; Tartakovsky, V. A. Russ. Chem. Rev. 2020, 89, 1–54. doi:10.1070/rcr4908 |
| 10. | Poręba, K.; Wietrzyk, J. Adv. Clin. Exp. Med. 2012, 21, 563–571. |
| 13. | Poręba, K.; Wietrzyk, J. Isoxazolo[4,5-b]pyridine derivative of the chemical name amide of 6-benzoyl-5,7-diphenyloisoxazolo[4,5-b]pyridine-3-carboxylic acid and antiproliferative activity and process for the preparation thereof. Pol. Patent 215605, Dec 31, 2013. |
| 10. | Poręba, K.; Wietrzyk, J. Adv. Clin. Exp. Med. 2012, 21, 563–571. |
| 13. | Poręba, K.; Wietrzyk, J. Isoxazolo[4,5-b]pyridine derivative of the chemical name amide of 6-benzoyl-5,7-diphenyloisoxazolo[4,5-b]pyridine-3-carboxylic acid and antiproliferative activity and process for the preparation thereof. Pol. Patent 215605, Dec 31, 2013. |
| 11. | Balog, J. A.; Huang, A.; Velaparthi, U.; Liu, P. Substituted bicyclic heteroaryl compounds. WO Patent WO2013049263, April 4, 2013. |
| 15. | Engers, D. W.; Blobaum, A. L.; Gogliotti, R. D.; Cheung, Y.-Y.; Salovich, J. M.; Garcia-Barrantes, P. M.; Daniels, J. S.; Morrison, R.; Jones, C. K.; Soars, M. G.; Zhuo, X.; Hurley, J.; Macor, J. E.; Bronson, J. J.; Conn, P. J.; Lindsley, C. W.; Niswender, C. M.; Hopkins, C. R. ACS Chem. Neurosci. 2016, 7, 1192–1200. doi:10.1021/acschemneuro.6b00035 |
| 16. | Conn, P. J.; Hopkins, C. R.; Lindsley, C. W.; Niswender, C. M.; Engers, D. W.; Panarese, J.; Bollinger, S.; Engers, J. Benzoisoxazole-substituted compounds as MGLUR4 allosteric potentiators, compositions, and methods of treating neurological dysfunction. WO Patent WO2016115282, July 21, 2016. |
| 17. | Yu, W.; Bulger, P. G.; Maloney, K. M. Green Chem. 2016, 18, 4941–4946. doi:10.1039/c6gc01125j |
| 18. | Tichenor, M. S.; Keith, J. M.; Jones, W. M.; Pierce, J. M.; Merit, J.; Hawryluk, N.; Seierstad, M.; Palmer, J. A.; Webb, M.; Karbarz, M. J.; Wilson, S. J.; Wennerholm, M. L.; Woestenborghs, F.; Beerens, D.; Luo, L.; Brown, S. M.; De Boeck, M.; Chaplan, S. R.; Breitenbucher, J. G. Bioorg. Med. Chem. Lett. 2012, 22, 7357–7362. doi:10.1016/j.bmcl.2012.10.076 |
| 19. | Hong, Y. R.; Kim, H. T.; Ro, S.; Cho, J. M.; Lee, S. H.; Kim, I. S.; Jung, Y. H. Bioorg. Med. Chem. Lett. 2014, 24, 3142–3145. doi:10.1016/j.bmcl.2014.05.003 |
| 33. | Madadi, N. R.; Penthala, N. R.; Howk, K.; Ketkar, A.; Eoff, R. L.; Borrelli, M. J.; Crooks, P. A. Eur. J. Med. Chem. 2015, 103, 123–132. doi:10.1016/j.ejmech.2015.08.041 |
| 12. | Rajanarendar, E.; Govardhan Reddy, K.; Ramakrishna, S.; Nagi Reddy, M.; Shireesha, B.; Durgaiah, G.; Reddy, Y. N. Bioorg. Med. Chem. Lett. 2012, 22, 6677–6680. doi:10.1016/j.bmcl.2012.08.098 |
| 8. | Thalji, R. K.; Raha, K.; Andreotti, D.; Checchia, A.; Cui, H.; Meneghelli, G.; Profeta, R.; Tonelli, F.; Tommasi, S.; Bakshi, T.; Donovan, B. T.; Howells, A.; Jain, S.; Nixon, C.; Quinque, G.; McCloskey, L.; Bax, B. D.; Neu, M.; Chan, P. F.; Stavenger, R. A. Bioorg. Med. Chem. Lett. 2019, 29, 1407–1412. doi:10.1016/j.bmcl.2019.03.029 |
| 20. | Hufnagel, B.; Zhu, W. F.; Franz, H. M.; Proschak, E.; Hernandez-Olmos, V. ChemistryOpen 2022, 11, e202200252. doi:10.1002/open.202200252 |
| 8. | Thalji, R. K.; Raha, K.; Andreotti, D.; Checchia, A.; Cui, H.; Meneghelli, G.; Profeta, R.; Tonelli, F.; Tommasi, S.; Bakshi, T.; Donovan, B. T.; Howells, A.; Jain, S.; Nixon, C.; Quinque, G.; McCloskey, L.; Bax, B. D.; Neu, M.; Chan, P. F.; Stavenger, R. A. Bioorg. Med. Chem. Lett. 2019, 29, 1407–1412. doi:10.1016/j.bmcl.2019.03.029 |
| 31. | George, D. T.; Gilman, J.; Hersh, J.; Thorsell, A.; Herion, D.; Geyer, C.; Peng, X.; Kielbasa, W.; Rawlings, R.; Brandt, J. E.; Gehlert, D. R.; Tauscher, J. T.; Hunt, S. P.; Hommer, D.; Heilig, M. Science 2008, 319, 1536–1539. doi:10.1126/science.1153813 |
| 11. | Balog, J. A.; Huang, A.; Velaparthi, U.; Liu, P. Substituted bicyclic heteroaryl compounds. WO Patent WO2013049263, April 4, 2013. |
| 14. | Gewald, K.; Bellmann, P.; Jänsch, H.-J. Liebigs Ann. Chem. 1980, 1623–1629. doi:10.1002/jlac.198019801018 |
| 32. | Němec, V.; Maier, L.; Berger, B.-T.; Chaikuad, A.; Drápela, S.; Souček, K.; Knapp, S.; Paruch, K. Eur. J. Med. Chem. 2021, 215, 113299. doi:10.1016/j.ejmech.2021.113299 |
© 2024 Nikol’skiy et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.