Abstract
We describe the use of bismuth(III) triflate as an efficient and environmentally friendly catalyst for the Nazarov reaction of aryl vinyl ketones, leading to the synthesis of 3-aryl-2-ethoxycarbonyl-1-indanones and 3-aryl-1-indanones. By changing the temperature and reaction time, it was possible to modulate the reactivity, allowing the synthesis of two distinct product classes (3-aryl-2-ethoxycarbonyl-1-indanones and 3-aryl-1-indanones) in good to excellent yield. The reaction did not require additives and was insensitive to both air and moisture. Preliminary biological evaluation of some indanones showed a promising profile against some human cancer line cells.
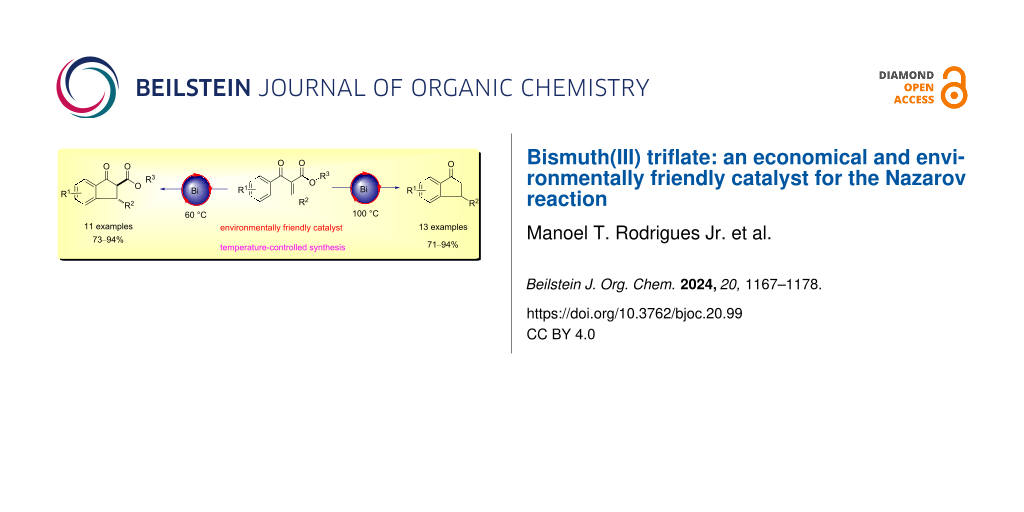
Graphical Abstract
Introduction
Natural products are the source of inspiration for several research groups that develop new synthetic methodologies. The chemistry of five-membered rings plays an important role in organic chemistry, both because of the wide occurrence in nature [1-6] and the broad spectrum of biological activities. Among the five-membered ring compounds, we find indanone (1, Figure 1). Within the class of indanones, we can highlight some interesting compounds. For example, nakiterpiosinone (2), which inhibits the growth of P388 mouse leukemia cells with an average inhibitory concentration (IC50) of 10 ng/mL, lepistatin A (3), along with two other new chlorinated analogs, that were isolated from Basidiomycete Lepista sordida culture, pauciflorol F (4), isolated from Vatica pauciflora, which is an important building block for the biosynthesis of bioactive polyphenols, in addition to having antiviral activity, indacrinone (5), which is related to ethacrynic acid and usually stimulates the reversible short-circuit current and the influx of sodium when applied to the epithelial surface of amphibian skin, and donepezil (6), a drug used to treat Alzheimer's disease [7-12].
Figure 1: Examples of different compounds containing the indanone moiety.
Figure 1: Examples of different compounds containing the indanone moiety.
The interest in the preparation of functionalized indanone derivatives has increased enormously, and many synthetic methods have been developed, including Friedel–Crafts cyclization reactions [13], cyclization of acetylenic derivatives [14], ring contractions and ring expansions [15], and the Nazarov reaction [16-20].
The Nazarov cyclization is one of the most versatile and simple methods for preparing indanones from aryl vinyl ketone derivatives [16-20]. The Nazarov reaction is classically formulated as a 4π conrotatory electrocyclization of a pentadienyl cation [1-12]. Until the past decade, the conditions used for the Nazarov reaction generally involved the use of a stoichiometric amount of a strong Lewis acid (e.g., BF3, TiCl4, SnCl4, AlCl3) in relation to the divinyl ketone derivative [21-23]. However, Dhoro and Tius demonstrated that weak acids could also be used as efficient catalysts for the Nazarov reaction [24].
In this context, some research groups developed methodologies that allowed the use of a catalytic amount of Lewis acid. By using more reactive divinyl ketone derivatives, the electrocyclization reaction could be mediated by weaker Lewis acids, and consequently a catalytic amount of them could be used. The first example of a catalytic version of the Nazarov cyclization was reported by Denmark and Jones [25-31]. They found that a substoichiometric amount of FeCl3 (40–50 mol %) promoted the cyclization of silylated derivatives efficiently. However, when 10 mol % was used, the conversion was poor. Denmark’s and Jones’s pioneering work was used as inspiration for the development of catalytic methodologies for this reaction. In 2004, Lang and Trauner described the first asymmetric catalytic Nazarov reaction [32]. In recent years, several strategies were reported employing different Lewis acids, such as, AuCl3/AgSbF6, Cu(II), In(OTf)3, Ir(III), Al(III), Sc(OTf)3/LiClO4, In(OTf)3/diphenylphosphoric acid (DPP), Fe(OTf)3/(CF3)2PhB(OH)2, iodine [33-43], and other strategies [44,45].
Although methodologies involving catalysis by Lewis acids are very efficient, including asymmetric versions of the Nazarov reaction, the experimental protocols are quite laborious in most cases, requiring low temperature, an inert atmosphere, or the use of Lewis acids sensitive to moisture [2,27,28,30,31,33,34,36,40-43,46-49].
Despite the numerous reports on catalytic versions of the Nazarov reaction, few of them describe the use of bismuth salts as catalysts for Nazarov-type reactions [50,51] and none for the classical Nazarov reaction. With the growing environmental concern and the need to use green reagents, interest in the use of bismuth in organic synthesis has increased significantly, as is reflected by the large number of works dedicated to this topic [52-54]. In addition to the replacement of toxic heavy metals, the use of bismuth compounds to promote reactions has the advantages of low cost and insensitivity to water and air. As such, the handling does not require special experimental techniques, such as an inert atmosphere and anhydrous solvents [55]. The use of bismuth salts in organic synthesis has been reported for several transformations, such as epoxide opening [56], ketal formation and deprotection [57,58], Mannich reaction [59], intramolecular Sakurai cyclization [60], alcohol oxidation [61], aromatic hydrocarbon nitration [62], imine allylation [63], Knoevenagel condensation [64], Reformatsky reaction [65], azalactone synthesis [66], nitro reduction [67,68], epoxide rearrangement, thiourea guanylation, and others [69,70].
In this article, we describe a simple and direct protocol for the preparation of indanones through a classical Nazarov reaction catalyzed by bismuth(III) triflate. In addition to the synthetic simplicity, the moisture stability of bismuth triflate allows the protocol to be carried out under ambient atmospheric conditions.
Results and Discussion
Preparation of β-ketoesters
We initiated our studies with the preparation of the β-ketoesters, which were synthesized according to well-established protocols [71-74]. The β-ketoesters were obtained employing a sequence of two reactions, the formation of the benzylic alcohol derivative, through a Reformatsky reaction using In(0), followed by a pyridinium chlorochromate (PCC) oxidation, giving the β-ketoesters 7a–g in moderate to good yields. With the β-ketoesters prepared, we began the synthesis of the Knoevenagel derivatives. To do so, we employed an adapted protocol from the literature. Using 1.00 equiv of β-ketoester, 1.50 equiv of aldehyde, 0.60 equiv of acetic acid, and 0.25 equiv of piperidine, the desired products were obtained in good to excellent yields and E-selectivities (Figure 2) [71-74]. Various substrates (9aa–gc), containing electron-donating, electron-withdrawing, electroneutral groups, and heteroaryl units (2-thiophene and 3-benzothiophene) were obtained in good to excellent yields. For derivatives 9af,ag,bq, we observed the formation of an E/Z mixture of products that was inseparable by column chromatography.
Figure 2: Synthesis of unsaturated β-ketoesters (Knoevenagel derivatives). aIsolated yield after purification using silica gel column chromatography.
Figure 2: Synthesis of unsaturated β-ketoesters (Knoevenagel derivatives). aIsolated yield after purification...
Synthesis of 3-aryl-2-ethoxycarbonyl-1-indanones and 3-aryl-1-indanones
With the starting materials prepared, we began evaluating the use of bismuth salts to promote the Nazarov reaction, using models already studied in the literature [33-43]. We investigated several conditions, such as the type of catalyst, temperature, solvent, and amount of catalyst (Table 1). Our optimization studies began with the reaction of substrate 9aa with Bi(OTf)3 (10 mol %) in acetonitrile at room temperature (Table 1, entry 1). The desired product was obtained in 72% after 12 h. When the reaction was carried out at 40 °C for 8 h, the yield increased slightly to 76% (Table 1, entry 2). When the reaction was carried out at 60 °C, the yield increased to 93%, in addition to a marked decrease in the reaction time (Table 1, entry 3). Despite this excellent result, we continued to evaluate other catalysts to improve the yield and reaction time further. For this purpose, a series of Lewis acids, such as Bi(NO)3, BiBr3, BiCl3, Yt(OTf)3, Dy(OTf)3, ZrCl4, In(OTf)3, InCl3, and AlCl3 were selected as catalysts (Table 1, entries 5–13), and even after this screening, the best result still remained the one obtained with Bi(OTf)3. The Brønsted acids TFA and TsOH were also tested for the transformation but gave worse results (Table 1, entries 14 and 15). Once the catalyst was chosen, we investigated the influence of the solvent. For this purpose, we evaluated dichloroethane (DCE), dichloromethane (DCM), toluene, and tetrahydrofuran (THF) as solvents for the transformation (Table 1, entries 16–19), but acetonitrile remained the best solvent. Finally, we evaluated the amount of catalyst, using two different catalyst loadings (5 and 20 mol %), but these variations also did not improve the yield (Table 1, entries 20 and 21). After this screening, the optimum conditions employed 10 mol % of bismuth triflate (Bi(OTf)3) in acetonitrile at 60 °C (Table 1, entry 3).
Table 1: Optimization of the reaction conditions.
|
|
|||||
| entry | catalyst (mol %) | solvent | T (°C) | t (h) | yield (%)a |
| 1 | Bi(OTf)3 (10) | acetonitrile | 25 | 12 | 72 |
| 2 | Bi(OTf)3 (10) | acetonitrile | 40 | 8 | 76 |
| 3 | Bi(OTf)3 (10) | acetonitrile | 60 | 2 | 93 |
| 4 | Bi(OTf)3 (10) | acetonitrile | 70 | 2 | 88 |
| 5 | Bi(NO)3 (10) | acetonitrile | 60 | 24 | 15b |
| 6 | BiBr3 (10) | acetonitrile | 60 | 24 | 26b |
| 7 | BiCl3 (10) | acetonitrile | 60 | 12 | 24b |
| 8 | Yt(OTf)3(10) | acetonitrile | 60 | 24 | 15b |
| 9 | Dy(OTf)3 (10) | acetonitrile | 60 | 12 | 45 |
| 10 | ZrCl4 (10) | acetonitrile | 60 | 12 | 58 |
| 11 | In(OTf)3 (10) | acetonitrile | 60 | 2 | 71 |
| 12 | InCl3 (10) | acetonitrile | 60 | 12 | 30 |
| 13 | AlCl3 (10) | acetonitrile | 60 | 24 | 5b |
| 14 | TFA (10) | acetonitrile | 60 | 6 | 58 |
| 15 | TsOH (10) | acetonitrile | 60 | 2 | 65 |
| 16 | Bi(OTf)3 (10) | DCE | 60 | 1 | 65 |
| 17 | Bi(OTf)3 (10) | DCM | 60 | 1 | 73 |
| 18 | Bi(OTf)3 (10) | toluene | 60 | 1.5 | 67 |
| 19 | Bi(OTf)3 (10) | THF | 60 | 3 | 53 |
| 20 | Bi(OTf)3 (5) | acetonitrile | 60 | 2 | 87 |
| 21 | Bi(OTf)3 (20) | acetonitrile | 60 | 1.5 | 91 |
| 22 | TfOH (10) | acetonitrile | 60 | 1.5 | 83 |
| 23 | Bi(OTf)3 (10) | DCE | 60 | 1 | 35b |
aIsolated yield after purification using silica gel column chromatography. bRecovery of starting material.
With the optimized conditions in hand, we explored the substrate scope (Figure 3). In general, substrates with electron donor groups provided a better yield when compared to those obtained with electroneutral or electron-withdrawing groups. Particularly for substrates 9ai,fc,gc, there was no formation of the corresponding indanones even though the reaction remained at 60 °C for a longer time (48 h), but the starting materials could be recovered. For substrate 9ah, there was complete decomposition of the starting material, along with the formation of several byproducts.
Figure 3: Synthesis of 3-aryl-2-ethoxycarbonyl-1-indanones mediated by bismuth triflate. aIsolated yield after purification using silica gel column chromatography. bExtensive degradation. cRecovery of starting material. Basic protocol: The Knoevenagel product 9 (0.5 mmol), dry acetonitrile (2 mL), and Bi(OTf)3 (0.05 mmol) were added to a sealed tube. The reaction mixture was stirred at 60 °C and monitored by TLC.
Figure 3: Synthesis of 3-aryl-2-ethoxycarbonyl-1-indanones mediated by bismuth triflate. aIsolated yield afte...
Surprisingly, when using the optimized conditions for substrates 9dc,dl, we found that in addition to the products of interest, they also formed the decarboxylated products as inseparable mixtures. Given this result, we investigated milder conditions to avoid decarboxylation. In doing so, we reacted the substrate 9dc for 24 h at room temperature. Despite this long reaction time, we still observed formation of the decarboxylated derivative, and we could also partly recover the starting material (Table 2).
Table 2: Evaluation of the reactivity of 9dc,dl.
|
|
||
| R | yield (%)a | 10/11 ratiob |
| 3,4,5-(CH3O)3C6H2 (i.e.,9dc) | 77 | 8:2 |
| piperonyl (i.e., 9dl) | 75 | 7:3 |
aYield determined by 1H NMR analysis using dimethyl terephthalate as internal standard. bRatio determined by 1H NMR analysis.
Decarboxylation reactions of indanones have previously been described in the literature. In 2008, Itoh et al. investigated the Nazarov cyclization of 3-substituted thiophene derivatives. When carrying out the reactions at 60 °C for 24 h, the formation of a 9:1 mixture of the products 13 and 14 in 61% combined yield was observed (Scheme 1) [75]. Under more vigorous conditions (100 °C for 5 h), there was a slight increase in the yield of decarboxylated product 14. In 2010, Zhang et al. [76] developed a methodology catalyzed by In(OTf)3 for the synthesis of bicycles, and they also verified that decarboxylation occurred when the reaction remained at 80 °C for 6 h (Scheme 1). The same behavior was observed by France during the synthesis of the lilolidone nucleus [77] and by Jung in the stereoselective synthesis of podophyllotoxin derivatives (Scheme 1) [78]. Although the decarboxylation reaction of indanones has been observed and described in the literature, there are few extensive studies aimed at exploring this transformation. The only exception is a report by Rajesh and Prajapati from 2015 [79]. In this work, the authors aimed at obtaining substituted β,β-indanones, and the decarboxylation step was a mandatory part of the methodology, with no interest in controlling the process.
Scheme 1: Previous methods describing decarboxylation reactions of indanones and xanthenones.
Scheme 1: Previous methods describing decarboxylation reactions of indanones and xanthenones.
The indanone core is a privileged structure, as it is often found in a series of natural products and synthetic molecules with different biological activities [11,80,81]. In particular, 1-indanones substituted in position 3 are important synthons for some drugs and natural products [82-84]. A bibliographic survey revealed that some methods were developed for the synthesis of 3-aryl-1-indanones. In this context, due to the formation of the previously shown derivatives 11dc,dl, we decided to explore the Nazarov reaction–decarboxylation sequence catalyzed by Bi(OTf)3 to prepare variously substituted 3-aryl-1-indanones from substrate 9.
We initially investigated the behavior of substrate 9aa under the conditions established for the Nazarov cyclization (60 °C). However, despite the reaction remaining under these conditions for a long period (24 h), only partial decarboxylation of the Nazarov product was observed. Thus, we decided to increase the temperature to 100 °C, and the reaction was maintained under these conditions in a sealed tube for 12 h. To our delight, we obtained only the decarboxylated indanone in an excellent yield (93%). These conditions were chosen as the optimal conditions, and the scope for the synthesis of 3-aryl-1-indanones derivatives is summarized in Figure 4.
Figure 4: Controlled decarboxylation directed by bismuth triflate at 100 °C. Synthesis of 3-aryl-1-indanones. aIsolated yield after purification using silica gel column chromatography. bReaction performed at 60 °C. cFrom tert-butyl Knoevenagel derivative. Basic protocol: The Knoevenagel product 9 (0.5 mmol), dry acetonitrile (2 mL), and Bi(OTf)3 (0.05 mmol) were added to a sealed tube. The reaction mixture was stirred at 100 °C and monitored by TLC.
Figure 4: Controlled decarboxylation directed by bismuth triflate at 100 °C. Synthesis of 3-aryl-1-indanones. ...
Simply by controlling the reaction temperature, it was possible to obtain indanones with different substitution patterns. At the lower temperature (60 °C), 2,3-substituted indanones could be obtained, while at the higher temperature (100 °C), 3-substituted indanones were achieved. Under both conditions, virtually no product mixtures were observed.
Preliminary biological evaluation
To investigate the cytotoxic activity of the indanone derivatives, in total 20 compounds were tested at a concentration of 5 and 50 μg/mL for 72 h in a panel of four histologically unrelated tumor lines, HCT116 (colon adenocarcinoma), MCF7 (breast adenocarcinoma), SK-MEL-28 (melanoma), and NB4 (acute leukemia) by methylthiazol tetrazolium (MTT) assay, as previously described [85]. Among the tested cells, NB4 cells were the most sensitive ones, with 7 compounds (10aa,bk and 11aa,ad,ae,dc,bj) being active at 5 μg/mL, using a 75% cutoff inhibition at each concentration. On the other hand, MCF-7 and SK-MEL-28 cells were the most resistant ones, with no compound being active at the lower concentration (Figure 5). This evidence of selective cytotoxicity for a specific histological tumor subtype may drive further structure–activity relationship studies to identify indanone targets of pharmacological interest.
![[1860-5397-20-99-5]](/bjoc/content/figures/1860-5397-20-99-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Impact of indanone derivatives on cell viability of tumor cells. Cell viability was determined by MTT assay. Data is expressed as reduction in viability in relation to the vehicle, and the dotted line indicates 75% reduction in cell viability. Compounds that reduced cell viability by at least 75% at 5 µg/mL are highlighted in the graph.
Figure 5: Impact of indanone derivatives on cell viability of tumor cells. Cell viability was determined by M...
Conclusion
In summary, we developed a simple and efficient methodology for the Nazarov reaction of aryl vinyl ketones, leading to 3-aryl-2-ethoxycarbonyl-1-indanones and 3-aryl-1-indanones. The reactions were catalyzed by bismuth triflate, an environmentally friendly metal. By simply changing the temperature and reaction time, it was possible to modulate the reactivity. In this methodology, no additives were used, and the reaction was insensitive to both air and moisture. To the best of our knowledge, this study is unique in the sense that there are no previous reports on the use of bismuth triflate as catalyst for a classic Nazarov reaction. There is also no reported precedent for the preparation of indanones with different substitution patterns through simple control of the reaction temperature.
The initial biological profile of 20 indanones was assessed, revealing promising activity against certain human cancer cell lines in some cases. To enhance the anticancer potential of these compounds, it is imperative to carry out additional comprehensive studies.
Supporting Information
| Supporting Information File 1: Experimental section and copies of 1H and 13C NMR spectra of all new compounds. | ||
| Format: PDF | Size: 8.1 MB | Download |
Funding
The authors thank the São Paulo Foundation Science (FAPESP, #2018/02611-0, #2013/07600-3 and #2023/18007-3, to FC) for financial support. F. C. is also grateful to the Brazilian National Council for Scientific and Technological Development (CNPq) for financial support and a research fellowship (#306142/2022-8). This study was partly funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Brasil. H. S. (#2015/09205-0) thanks FAPESP for a Ph.D. fellowship. L. A. Z. thanks CNPq for his fellowship.
This study was also supported by the grants #2017/24993-0, #2019/23864-7, and #2015/17177-6, São Paulo Research Foundation (FAPESP) and grants #402587/2016-2, #306142022-8, and #407960/2023-6, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Williams, D. R.; Robinson, L. A.; Nevill, C. R.; Reddy, J. P. Angew. Chem., Int. Ed. 2007, 46, 915–918. doi:10.1002/anie.200603853
Return to citation in text: [1] [2] -
He, W.; Huang, J.; Sun, X.; Frontier, A. J. J. Am. Chem. Soc. 2008, 130, 300–308. doi:10.1021/ja0761986
Return to citation in text: [1] [2] [3] -
Wan, L.; Tius, M. A. Org. Lett. 2007, 9, 647–650. doi:10.1021/ol062919e
Return to citation in text: [1] [2] -
Harding, K. E.; Clement, K. S.; Tseng, C. Y. J. Org. Chem. 1990, 55, 4403–4410. doi:10.1021/jo00301a036
Return to citation in text: [1] [2] -
Tius, M. A.; Drake, D. J. Tetrahedron 1996, 52, 14651–14660. doi:10.1016/0040-4020(96)00913-1
Return to citation in text: [1] [2] -
Mateos, A. F.; Martín de la Nava, E. M.; González, R. R. J. Org. Chem. 2001, 66, 7632–7638. doi:10.1021/jo010449q
Return to citation in text: [1] [2] -
Teruya, T.; Nakagawa, S.; Koyama, T.; Arimoto, H.; Kita, M.; Uemura, D. Tetrahedron 2004, 60, 6989–6993. doi:10.1016/j.tet.2003.08.083
Return to citation in text: [1] [2] -
Kang, H.-S.; Ji, S.-A.; Park, S.-H.; Kim, J.-P. Phytochemistry 2017, 143, 111–114. doi:10.1016/j.phytochem.2017.08.003
Return to citation in text: [1] [2] -
Faiz, S.; Yousaf, M.; Zahoor, A. F.; Naqvi, S. A. R.; Irfan, A.; Zaman, G. Synth. Commun. 2017, 47, 1121–1135. doi:10.1080/00397911.2017.1303510
Return to citation in text: [1] [2] -
Korabecny, J.; Spilovska, K.; Mezeiova, E.; Benek, O.; Juza, R.; Kaping, D.; Soukup, O. Curr. Med. Chem. 2019, 26, 5625–5648. doi:10.2174/0929867325666180517094023
Return to citation in text: [1] [2] -
Patil, S. A.; Patil, R.; Patil, S. A. Eur. J. Med. Chem. 2017, 138, 182–198. doi:10.1016/j.ejmech.2017.06.032
Return to citation in text: [1] [2] [3] -
Li, M.; Xia, L. Chem. Biol. Drug Des. 2007, 70, 461–464. doi:10.1111/j.1747-0285.2007.00581.x
Return to citation in text: [1] [2] -
Turek, M.; Szczęsna, D.; Koprowski, M.; Bałczewski, P. Beilstein J. Org. Chem. 2017, 13, 451–494. doi:10.3762/bjoc.13.48
Return to citation in text: [1] -
Das, S.; Dutta, A. RSC Adv. 2022, 12, 33365–33402. doi:10.1039/d2ra06635a
Return to citation in text: [1] -
Siqueira, F. A.; Ishikawa, E. E.; Fogaça, A.; Faccio, A. T.; Carneiro, V. M. T.; Soares, R. R. S.; Utaka, A.; Tébéka, I. R. M.; Bielawski, M.; Olofsson, B.; Silva, L. F., Jr. J. Braz. Chem. Soc. 2011, 22, 1795–1807. doi:10.1590/s0103-50532011000900024
Return to citation in text: [1] -
Pellissier, H. Tetrahedron 2005, 61, 6479–6517. doi:10.1016/j.tet.2005.04.014
Return to citation in text: [1] [2] -
Tius, M. A. Eur. J. Org. Chem. 2005, 2193–2206. doi:10.1002/ejoc.200500005
Return to citation in text: [1] [2] -
Vinogradov, M. G.; Turova, O. V.; Zlotin, S. G. Org. Biomol. Chem. 2017, 15, 8245–8269. doi:10.1039/c7ob01981e
Return to citation in text: [1] [2] -
Wenz, D. R.; Read de Alaniz, J. Eur. J. Org. Chem. 2015, 23–37. doi:10.1002/ejoc.201402825
Return to citation in text: [1] [2] -
Vaidya, T.; Eisenberg, R.; Frontier, A. J. ChemCatChem 2011, 3, 1531–1548. doi:10.1002/cctc.201100137
Return to citation in text: [1] [2] -
Frontier, A. J.; Collison, C. Tetrahedron 2005, 61, 7577–7606. doi:10.1016/j.tet.2005.05.019
Return to citation in text: [1] -
Frontier, A. J.; Hernandez, J. J. Acc. Chem. Res. 2020, 53, 1822–1832. doi:10.1021/acs.accounts.0c00284
Return to citation in text: [1] -
Yadykov, A. V.; Shirinian, V. Z. Adv. Synth. Catal. 2020, 362, 702–723. doi:10.1002/adsc.201901001
Return to citation in text: [1] -
Dhoro, F.; Tius, M. A. J. Am. Chem. Soc. 2005, 127, 12472–12473. doi:10.1021/ja053393g
Return to citation in text: [1] -
Jones, T. K.; Denmark, S. E. Helv. Chim. Acta 1983, 66, 2377–2396. doi:10.1002/hlca.19830660802
Return to citation in text: [1] -
Larini, P.; Guarna, A.; Occhiato, E. G. Org. Lett. 2006, 8, 781–784. doi:10.1021/ol053071h
Return to citation in text: [1] -
Fujiwara, M.; Kawatsura, M.; Hayase, S.; Nanjo, M.; Itoh, T. Adv. Synth. Catal. 2009, 351, 123–128. doi:10.1002/adsc.200800702
Return to citation in text: [1] [2] -
Rieder, C. J.; Winberg, K. J.; West, F. G. J. Am. Chem. Soc. 2009, 131, 7504–7505. doi:10.1021/ja9023226
Return to citation in text: [1] [2] -
Bitar, A. Y.; Frontier, A. J. Org. Lett. 2009, 11, 49–52. doi:10.1021/ol802329y
Return to citation in text: [1] -
Liang, G.; Gradl, S. N.; Trauner, D. Org. Lett. 2003, 5, 4931–4934. doi:10.1021/ol036019z
Return to citation in text: [1] [2] -
Malona, J. A.; Colbourne, J. M.; Frontier, A. J. Org. Lett. 2006, 8, 5661–5664. doi:10.1021/ol062403v
Return to citation in text: [1] [2] -
Liang, G.; Trauner, D. J. Am. Chem. Soc. 2004, 126, 9544–9545. doi:10.1021/ja0476664
Return to citation in text: [1] -
Janka, M.; He, W.; Frontier, A. J.; Eisenberg, R. J. Am. Chem. Soc. 2004, 126, 6864–6865. doi:10.1021/ja049643v
Return to citation in text: [1] [2] [3] -
Vaidya, T.; Atesin, A. C.; Herrick, I. R.; Frontier, A. J.; Eisenberg, R. Angew. Chem., Int. Ed. 2010, 49, 3363–3366. doi:10.1002/anie.201000100
Return to citation in text: [1] [2] [3] -
Marcus, A. P.; Lee, A. S.; Davis, R. L.; Tantillo, D. J.; Sarpong, R. Angew. Chem., Int. Ed. 2008, 47, 6379–6383. doi:10.1002/anie.200801542
Return to citation in text: [1] [2] -
Jin, T.; Yamamoto, Y. Org. Lett. 2008, 10, 3137–3139. doi:10.1021/ol801265s
Return to citation in text: [1] [2] [3] -
Koenig, J. J.; Arndt, T.; Gildemeister, N.; Neudörfl, J.-M.; Breugst, M. J. Org. Chem. 2019, 84, 7587–7605. doi:10.1021/acs.joc.9b01083
Return to citation in text: [1] [2] -
Xing, S.; Xia, H.; Guo, J.; Zou, C.; Gao, T.; Wang, K.; Zhu, B.; Pei, M.; Bai, M. J. Org. Chem. 2019, 84, 8984–8997. doi:10.1021/acs.joc.9b00876
Return to citation in text: [1] [2] -
Süsse, L.; Vogler, M.; Mewald, M.; Kemper, B.; Irran, E.; Oestreich, M. Angew. Chem., Int. Ed. 2018, 57, 11441–11444. doi:10.1002/anie.201806011
Return to citation in text: [1] [2] -
Zhou, X.; Zhao, Y.; Cao, Y.; He, L. Adv. Synth. Catal. 2017, 359, 3325–3331. doi:10.1002/adsc.201700820
Return to citation in text: [1] [2] [3] -
Wang, G.-P.; Chen, M.-Q.; Zhu, S.-F.; Zhou, Q.-L. Chem. Sci. 2017, 8, 7197–7202. doi:10.1039/c7sc03183a
Return to citation in text: [1] [2] [3] -
Krieger, J.; Smeilus, T.; Schackow, O.; Giannis, A. Chem. – Eur. J. 2017, 23, 5000–5004. doi:10.1002/chem.201701008
Return to citation in text: [1] [2] [3] -
Shirinian, V. Z.; Lvov, A. G.; Yadykov, A. V.; Yaminova, L. V.; Kachala, V. V.; Markosyan, A. I. Org. Lett. 2016, 18, 6260–6263. doi:10.1021/acs.orglett.6b03023
Return to citation in text: [1] [2] [3] -
Xi, Z.-G.; Zhu, L.; Luo, S.; Cheng, J.-P. J. Org. Chem. 2013, 78, 606–613. doi:10.1021/jo302451b
Return to citation in text: [1] -
Takeda, T.; Harada, S.; Nishida, A. Org. Lett. 2015, 17, 5184–5187. doi:10.1021/acs.orglett.5b02497
Return to citation in text: [1] -
Batson, W. A.; Sethumadhavan, D.; Tius, M. A. Org. Lett. 2005, 7, 2771–2774. doi:10.1021/ol050970x
Return to citation in text: [1] -
Cordier, P.; Aubert, C.; Malacria, M.; Lacôte, E.; Gandon, V. Angew. Chem., Int. Ed. 2009, 48, 8757–8760. doi:10.1002/anie.200903675
Return to citation in text: [1] -
Rieder, C. J.; Winberg, K. J.; West, F. G. J. Org. Chem. 2011, 76, 50–56. doi:10.1021/jo101497f
Return to citation in text: [1] -
Ondet, P.; Lemière, G.; Duñach, E. Eur. J. Org. Chem. 2017, 761–780. doi:10.1002/ejoc.201600937
Return to citation in text: [1] -
Lempenauer, L.; Duñach, E.; Lemière, G. Chem. – Eur. J. 2017, 23, 10285–10288. doi:10.1002/chem.201702601
Return to citation in text: [1] -
Ondet, P.; Joffrin, A.; Diaf, I.; Lemière, G.; Duñach, E. Org. Lett. 2015, 17, 1002–1005. doi:10.1021/acs.orglett.5b00110
Return to citation in text: [1] -
Leonard, N. M.; Wieland, L. C.; Mohan, R. S. Tetrahedron 2002, 58, 8373–8397. doi:10.1016/s0040-4020(02)01000-1
Return to citation in text: [1] -
Bothwell, J. M.; Krabbe, S. W.; Mohan, R. S. Chem. Soc. Rev. 2011, 40, 4649–4707. doi:10.1039/c0cs00206b
Return to citation in text: [1] -
Kakde, B. N.; Kumar, N.; Mondal, P. K.; Bisai, A. Org. Lett. 2016, 18, 1752–1755. doi:10.1021/acs.orglett.6b00446
Return to citation in text: [1] -
Aggen, D. H.; Arnold, J. N.; Hayes, P. D.; Smoter, N. J.; Mohan, R. S. Tetrahedron 2004, 60, 3675–3679. doi:10.1016/j.tet.2004.02.046
Return to citation in text: [1] -
Ollevier, T.; Lavie-Compin, G. Tetrahedron Lett. 2004, 45, 49–52. doi:10.1016/j.tetlet.2003.10.129
Return to citation in text: [1] -
Leonard, N. M.; Oswald, M. C.; Freiberg, D. A.; Nattier, B. A.; Smith, R. C.; Mohan, R. S. J. Org. Chem. 2002, 67, 5202–5207. doi:10.1021/jo0258249
Return to citation in text: [1] -
Carrigan, M. D.; Sarapa, D.; Smith, R. C.; Wieland, L. C.; Mohan, R. S. J. Org. Chem. 2002, 67, 1027–1030. doi:10.1021/jo016180s
Return to citation in text: [1] -
Ollevier, T.; Nadeau, E. J. Org. Chem. 2004, 69, 9292–9295. doi:10.1021/jo048617c
Return to citation in text: [1] -
Leroy, B.; Markó, I. E. Org. Lett. 2002, 4, 47–50. doi:10.1021/ol016863u
Return to citation in text: [1] -
Samajdar, S.; Becker, F. F.; Banik, B. K. Synth. Commun. 2001, 31, 2691–2695. doi:10.1081/scc-100105397
Return to citation in text: [1] -
Cornélis, A.; Delaude, L.; Gerstmans, A.; Laszlo, P. Tetrahedron Lett. 1988, 29, 5657–5660. doi:10.1016/s0040-4039(00)80837-0
Return to citation in text: [1] -
Tanaka, H.; Nakahara, T.; Dhimane, H.; Torii, S. Tetrahedron Lett. 1989, 30, 4161–4164. doi:10.1016/s0040-4039(00)99348-1
Return to citation in text: [1] -
Prajapati, D.; Sandhu, J. S. Chem. Lett. 1992, 21, 1945–1946. doi:10.1246/cl.1992.1945
Return to citation in text: [1] -
Shen, Z.; Zhang, J.; Zou, H.; Yang, M. Tetrahedron Lett. 1997, 38, 2733–2736. doi:10.1016/s0040-4039(97)00456-5
Return to citation in text: [1] -
Monk, K. A.; Sarapa, D.; Mohan, R. S. Synth. Commun. 2000, 30, 3167–3170. doi:10.1080/00397910008086926
Return to citation in text: [1] -
Borah, H. N.; Prajapati, D.; Sandhu, J. S.; Ghosh, A. C. Tetrahedron Lett. 1994, 35, 3167–3170. doi:10.1016/s0040-4039(00)76858-4
Return to citation in text: [1] -
Ren, P.; Pan, S.; Dong, T.; Wu, S. Synth. Commun. 1996, 26, 3903–3908. doi:10.1080/00397919608003810
Return to citation in text: [1] -
Bhatia, K. A.; Eash, K. J.; Leonard, N. M.; Oswald, M. C.; Mohan, R. S. Tetrahedron Lett. 2001, 42, 8129–8132. doi:10.1016/s0040-4039(01)01519-2
Return to citation in text: [1] -
Cunha, S.; Rodrigues, M. T., Jr. Tetrahedron Lett. 2006, 47, 6955–6956. doi:10.1016/j.tetlet.2006.07.138
Return to citation in text: [1] -
Poisson, T.; Belhomme, M.-C.; Pannecoucke, X. J. Org. Chem. 2012, 77, 9277–9285. doi:10.1021/jo301873y
Return to citation in text: [1] [2] -
Chen, X.; Zhang, C.; Wu, H.; Yu, X.; Su, W.; Cheng, J. Synthesis 2007, 3233–3239. doi:10.1055/s-2007-990799
Return to citation in text: [1] [2] -
Corey, E. J.; Suggs, J. W. Tetrahedron Lett. 1975, 16, 2647–2650. doi:10.1016/s0040-4039(00)75204-x
Return to citation in text: [1] [2] -
Davies, J.; Leonori, D. Chem. Commun. 2014, 50, 15171–15174. doi:10.1039/c4cc06501h
Return to citation in text: [1] [2] -
Kawatsura, M.; Higuchi, Y.; Hayase, S.; Nanjo, M.; Itoh, T. Synlett 2008, 1009–1012. doi:10.1055/s-2008-1072503
Return to citation in text: [1] -
Liu, L.; Wei, L.; Lu, Y.; Zhang, J. Chem. – Eur. J. 2010, 16, 11813–11817. doi:10.1002/chem.201001729
Return to citation in text: [1] -
Patil, D. V.; Cavitt, M. A.; Grzybowski, P.; France, S. Chem. Commun. 2012, 48, 10337–10339. doi:10.1039/c2cc34650h
Return to citation in text: [1] -
Jung, M. E.; Lam, P. Y.-S.; Mansuri, M. M.; Speltz, L. M. J. Org. Chem. 1985, 50, 1087–1105. doi:10.1021/jo00207a034
Return to citation in text: [1] -
Rajesh, N.; Prajapati, D. Chem. Commun. 2015, 51, 3347–3350. doi:10.1039/c4cc09799h
Return to citation in text: [1] -
Lawrence, N. J.; Armitage, E. S. M.; Greedy, B.; Cook, D.; Ducki, S.; McGown, A. T. Tetrahedron Lett. 2006, 47, 1637–1640. doi:10.1016/j.tetlet.2005.12.110
Return to citation in text: [1] -
Prakasham, A. P.; Saxena, A. K.; Luqman, S.; Chanda, D.; Kaur, T.; Gupta, A.; Yadav, D. K.; Chanotiya, C. S.; Shanker, K.; Khan, F.; Negi, A. S. Bioorg. Med. Chem. 2012, 20, 3049–3057. doi:10.1016/j.bmc.2012.02.057
Return to citation in text: [1] -
Bogeso, K. P.; Christensen, A. V.; Hyttel, J.; Liljefors, T. J. Med. Chem. 1985, 28, 1817–1828. doi:10.1021/jm00150a012
Return to citation in text: [1] -
Lee, B. H.; Choi, Y. L.; Shin, S.; Heo, J.-N. J. Org. Chem. 2011, 76, 6611–6618. doi:10.1021/jo2009164
Return to citation in text: [1] -
Yu, Y.-N.; Xu, M.-H. J. Org. Chem. 2013, 78, 2736–2741. doi:10.1021/jo302656s
Return to citation in text: [1] -
Vicari, H. P.; Lima, K.; Gomes, R. d. C.; Fernandes, D. C.; da Silva, J. C. L.; Rodrigues Junior, M. T.; Barroso de Oliveira, A. S.; dos Santos, R. N.; Andricopulo, A. D.; Coelho, F.; Costa-Lotufo, L. V.; Machado-Neto, J. A. Eur. J. Pharmacol. 2021, 894, 173853. doi:10.1016/j.ejphar.2021.173853
Return to citation in text: [1]
| 66. | Monk, K. A.; Sarapa, D.; Mohan, R. S. Synth. Commun. 2000, 30, 3167–3170. doi:10.1080/00397910008086926 |
| 67. | Borah, H. N.; Prajapati, D.; Sandhu, J. S.; Ghosh, A. C. Tetrahedron Lett. 1994, 35, 3167–3170. doi:10.1016/s0040-4039(00)76858-4 |
| 68. | Ren, P.; Pan, S.; Dong, T.; Wu, S. Synth. Commun. 1996, 26, 3903–3908. doi:10.1080/00397919608003810 |
| 69. | Bhatia, K. A.; Eash, K. J.; Leonard, N. M.; Oswald, M. C.; Mohan, R. S. Tetrahedron Lett. 2001, 42, 8129–8132. doi:10.1016/s0040-4039(01)01519-2 |
| 70. | Cunha, S.; Rodrigues, M. T., Jr. Tetrahedron Lett. 2006, 47, 6955–6956. doi:10.1016/j.tetlet.2006.07.138 |
| 1. | Williams, D. R.; Robinson, L. A.; Nevill, C. R.; Reddy, J. P. Angew. Chem., Int. Ed. 2007, 46, 915–918. doi:10.1002/anie.200603853 |
| 2. | He, W.; Huang, J.; Sun, X.; Frontier, A. J. J. Am. Chem. Soc. 2008, 130, 300–308. doi:10.1021/ja0761986 |
| 3. | Wan, L.; Tius, M. A. Org. Lett. 2007, 9, 647–650. doi:10.1021/ol062919e |
| 4. | Harding, K. E.; Clement, K. S.; Tseng, C. Y. J. Org. Chem. 1990, 55, 4403–4410. doi:10.1021/jo00301a036 |
| 5. | Tius, M. A.; Drake, D. J. Tetrahedron 1996, 52, 14651–14660. doi:10.1016/0040-4020(96)00913-1 |
| 6. | Mateos, A. F.; Martín de la Nava, E. M.; González, R. R. J. Org. Chem. 2001, 66, 7632–7638. doi:10.1021/jo010449q |
| 15. | Siqueira, F. A.; Ishikawa, E. E.; Fogaça, A.; Faccio, A. T.; Carneiro, V. M. T.; Soares, R. R. S.; Utaka, A.; Tébéka, I. R. M.; Bielawski, M.; Olofsson, B.; Silva, L. F., Jr. J. Braz. Chem. Soc. 2011, 22, 1795–1807. doi:10.1590/s0103-50532011000900024 |
| 2. | He, W.; Huang, J.; Sun, X.; Frontier, A. J. J. Am. Chem. Soc. 2008, 130, 300–308. doi:10.1021/ja0761986 |
| 27. | Fujiwara, M.; Kawatsura, M.; Hayase, S.; Nanjo, M.; Itoh, T. Adv. Synth. Catal. 2009, 351, 123–128. doi:10.1002/adsc.200800702 |
| 28. | Rieder, C. J.; Winberg, K. J.; West, F. G. J. Am. Chem. Soc. 2009, 131, 7504–7505. doi:10.1021/ja9023226 |
| 30. | Liang, G.; Gradl, S. N.; Trauner, D. Org. Lett. 2003, 5, 4931–4934. doi:10.1021/ol036019z |
| 31. | Malona, J. A.; Colbourne, J. M.; Frontier, A. J. Org. Lett. 2006, 8, 5661–5664. doi:10.1021/ol062403v |
| 33. | Janka, M.; He, W.; Frontier, A. J.; Eisenberg, R. J. Am. Chem. Soc. 2004, 126, 6864–6865. doi:10.1021/ja049643v |
| 34. | Vaidya, T.; Atesin, A. C.; Herrick, I. R.; Frontier, A. J.; Eisenberg, R. Angew. Chem., Int. Ed. 2010, 49, 3363–3366. doi:10.1002/anie.201000100 |
| 36. | Jin, T.; Yamamoto, Y. Org. Lett. 2008, 10, 3137–3139. doi:10.1021/ol801265s |
| 40. | Zhou, X.; Zhao, Y.; Cao, Y.; He, L. Adv. Synth. Catal. 2017, 359, 3325–3331. doi:10.1002/adsc.201700820 |
| 41. | Wang, G.-P.; Chen, M.-Q.; Zhu, S.-F.; Zhou, Q.-L. Chem. Sci. 2017, 8, 7197–7202. doi:10.1039/c7sc03183a |
| 42. | Krieger, J.; Smeilus, T.; Schackow, O.; Giannis, A. Chem. – Eur. J. 2017, 23, 5000–5004. doi:10.1002/chem.201701008 |
| 43. | Shirinian, V. Z.; Lvov, A. G.; Yadykov, A. V.; Yaminova, L. V.; Kachala, V. V.; Markosyan, A. I. Org. Lett. 2016, 18, 6260–6263. doi:10.1021/acs.orglett.6b03023 |
| 46. | Batson, W. A.; Sethumadhavan, D.; Tius, M. A. Org. Lett. 2005, 7, 2771–2774. doi:10.1021/ol050970x |
| 47. | Cordier, P.; Aubert, C.; Malacria, M.; Lacôte, E.; Gandon, V. Angew. Chem., Int. Ed. 2009, 48, 8757–8760. doi:10.1002/anie.200903675 |
| 48. | Rieder, C. J.; Winberg, K. J.; West, F. G. J. Org. Chem. 2011, 76, 50–56. doi:10.1021/jo101497f |
| 49. | Ondet, P.; Lemière, G.; Duñach, E. Eur. J. Org. Chem. 2017, 761–780. doi:10.1002/ejoc.201600937 |
| 78. | Jung, M. E.; Lam, P. Y.-S.; Mansuri, M. M.; Speltz, L. M. J. Org. Chem. 1985, 50, 1087–1105. doi:10.1021/jo00207a034 |
| 50. | Lempenauer, L.; Duñach, E.; Lemière, G. Chem. – Eur. J. 2017, 23, 10285–10288. doi:10.1002/chem.201702601 |
| 51. | Ondet, P.; Joffrin, A.; Diaf, I.; Lemière, G.; Duñach, E. Org. Lett. 2015, 17, 1002–1005. doi:10.1021/acs.orglett.5b00110 |
| 79. | Rajesh, N.; Prajapati, D. Chem. Commun. 2015, 51, 3347–3350. doi:10.1039/c4cc09799h |
| 13. | Turek, M.; Szczęsna, D.; Koprowski, M.; Bałczewski, P. Beilstein J. Org. Chem. 2017, 13, 451–494. doi:10.3762/bjoc.13.48 |
| 33. | Janka, M.; He, W.; Frontier, A. J.; Eisenberg, R. J. Am. Chem. Soc. 2004, 126, 6864–6865. doi:10.1021/ja049643v |
| 34. | Vaidya, T.; Atesin, A. C.; Herrick, I. R.; Frontier, A. J.; Eisenberg, R. Angew. Chem., Int. Ed. 2010, 49, 3363–3366. doi:10.1002/anie.201000100 |
| 35. | Marcus, A. P.; Lee, A. S.; Davis, R. L.; Tantillo, D. J.; Sarpong, R. Angew. Chem., Int. Ed. 2008, 47, 6379–6383. doi:10.1002/anie.200801542 |
| 36. | Jin, T.; Yamamoto, Y. Org. Lett. 2008, 10, 3137–3139. doi:10.1021/ol801265s |
| 37. | Koenig, J. J.; Arndt, T.; Gildemeister, N.; Neudörfl, J.-M.; Breugst, M. J. Org. Chem. 2019, 84, 7587–7605. doi:10.1021/acs.joc.9b01083 |
| 38. | Xing, S.; Xia, H.; Guo, J.; Zou, C.; Gao, T.; Wang, K.; Zhu, B.; Pei, M.; Bai, M. J. Org. Chem. 2019, 84, 8984–8997. doi:10.1021/acs.joc.9b00876 |
| 39. | Süsse, L.; Vogler, M.; Mewald, M.; Kemper, B.; Irran, E.; Oestreich, M. Angew. Chem., Int. Ed. 2018, 57, 11441–11444. doi:10.1002/anie.201806011 |
| 40. | Zhou, X.; Zhao, Y.; Cao, Y.; He, L. Adv. Synth. Catal. 2017, 359, 3325–3331. doi:10.1002/adsc.201700820 |
| 41. | Wang, G.-P.; Chen, M.-Q.; Zhu, S.-F.; Zhou, Q.-L. Chem. Sci. 2017, 8, 7197–7202. doi:10.1039/c7sc03183a |
| 42. | Krieger, J.; Smeilus, T.; Schackow, O.; Giannis, A. Chem. – Eur. J. 2017, 23, 5000–5004. doi:10.1002/chem.201701008 |
| 43. | Shirinian, V. Z.; Lvov, A. G.; Yadykov, A. V.; Yaminova, L. V.; Kachala, V. V.; Markosyan, A. I. Org. Lett. 2016, 18, 6260–6263. doi:10.1021/acs.orglett.6b03023 |
| 76. | Liu, L.; Wei, L.; Lu, Y.; Zhang, J. Chem. – Eur. J. 2010, 16, 11813–11817. doi:10.1002/chem.201001729 |
| 7. | Teruya, T.; Nakagawa, S.; Koyama, T.; Arimoto, H.; Kita, M.; Uemura, D. Tetrahedron 2004, 60, 6989–6993. doi:10.1016/j.tet.2003.08.083 |
| 8. | Kang, H.-S.; Ji, S.-A.; Park, S.-H.; Kim, J.-P. Phytochemistry 2017, 143, 111–114. doi:10.1016/j.phytochem.2017.08.003 |
| 9. | Faiz, S.; Yousaf, M.; Zahoor, A. F.; Naqvi, S. A. R.; Irfan, A.; Zaman, G. Synth. Commun. 2017, 47, 1121–1135. doi:10.1080/00397911.2017.1303510 |
| 10. | Korabecny, J.; Spilovska, K.; Mezeiova, E.; Benek, O.; Juza, R.; Kaping, D.; Soukup, O. Curr. Med. Chem. 2019, 26, 5625–5648. doi:10.2174/0929867325666180517094023 |
| 11. | Patil, S. A.; Patil, R.; Patil, S. A. Eur. J. Med. Chem. 2017, 138, 182–198. doi:10.1016/j.ejmech.2017.06.032 |
| 12. | Li, M.; Xia, L. Chem. Biol. Drug Des. 2007, 70, 461–464. doi:10.1111/j.1747-0285.2007.00581.x |
| 44. | Xi, Z.-G.; Zhu, L.; Luo, S.; Cheng, J.-P. J. Org. Chem. 2013, 78, 606–613. doi:10.1021/jo302451b |
| 45. | Takeda, T.; Harada, S.; Nishida, A. Org. Lett. 2015, 17, 5184–5187. doi:10.1021/acs.orglett.5b02497 |
| 77. | Patil, D. V.; Cavitt, M. A.; Grzybowski, P.; France, S. Chem. Commun. 2012, 48, 10337–10339. doi:10.1039/c2cc34650h |
| 21. | Frontier, A. J.; Collison, C. Tetrahedron 2005, 61, 7577–7606. doi:10.1016/j.tet.2005.05.019 |
| 22. | Frontier, A. J.; Hernandez, J. J. Acc. Chem. Res. 2020, 53, 1822–1832. doi:10.1021/acs.accounts.0c00284 |
| 23. | Yadykov, A. V.; Shirinian, V. Z. Adv. Synth. Catal. 2020, 362, 702–723. doi:10.1002/adsc.201901001 |
| 25. | Jones, T. K.; Denmark, S. E. Helv. Chim. Acta 1983, 66, 2377–2396. doi:10.1002/hlca.19830660802 |
| 26. | Larini, P.; Guarna, A.; Occhiato, E. G. Org. Lett. 2006, 8, 781–784. doi:10.1021/ol053071h |
| 27. | Fujiwara, M.; Kawatsura, M.; Hayase, S.; Nanjo, M.; Itoh, T. Adv. Synth. Catal. 2009, 351, 123–128. doi:10.1002/adsc.200800702 |
| 28. | Rieder, C. J.; Winberg, K. J.; West, F. G. J. Am. Chem. Soc. 2009, 131, 7504–7505. doi:10.1021/ja9023226 |
| 29. | Bitar, A. Y.; Frontier, A. J. Org. Lett. 2009, 11, 49–52. doi:10.1021/ol802329y |
| 30. | Liang, G.; Gradl, S. N.; Trauner, D. Org. Lett. 2003, 5, 4931–4934. doi:10.1021/ol036019z |
| 31. | Malona, J. A.; Colbourne, J. M.; Frontier, A. J. Org. Lett. 2006, 8, 5661–5664. doi:10.1021/ol062403v |
| 33. | Janka, M.; He, W.; Frontier, A. J.; Eisenberg, R. J. Am. Chem. Soc. 2004, 126, 6864–6865. doi:10.1021/ja049643v |
| 34. | Vaidya, T.; Atesin, A. C.; Herrick, I. R.; Frontier, A. J.; Eisenberg, R. Angew. Chem., Int. Ed. 2010, 49, 3363–3366. doi:10.1002/anie.201000100 |
| 35. | Marcus, A. P.; Lee, A. S.; Davis, R. L.; Tantillo, D. J.; Sarpong, R. Angew. Chem., Int. Ed. 2008, 47, 6379–6383. doi:10.1002/anie.200801542 |
| 36. | Jin, T.; Yamamoto, Y. Org. Lett. 2008, 10, 3137–3139. doi:10.1021/ol801265s |
| 37. | Koenig, J. J.; Arndt, T.; Gildemeister, N.; Neudörfl, J.-M.; Breugst, M. J. Org. Chem. 2019, 84, 7587–7605. doi:10.1021/acs.joc.9b01083 |
| 38. | Xing, S.; Xia, H.; Guo, J.; Zou, C.; Gao, T.; Wang, K.; Zhu, B.; Pei, M.; Bai, M. J. Org. Chem. 2019, 84, 8984–8997. doi:10.1021/acs.joc.9b00876 |
| 39. | Süsse, L.; Vogler, M.; Mewald, M.; Kemper, B.; Irran, E.; Oestreich, M. Angew. Chem., Int. Ed. 2018, 57, 11441–11444. doi:10.1002/anie.201806011 |
| 40. | Zhou, X.; Zhao, Y.; Cao, Y.; He, L. Adv. Synth. Catal. 2017, 359, 3325–3331. doi:10.1002/adsc.201700820 |
| 41. | Wang, G.-P.; Chen, M.-Q.; Zhu, S.-F.; Zhou, Q.-L. Chem. Sci. 2017, 8, 7197–7202. doi:10.1039/c7sc03183a |
| 42. | Krieger, J.; Smeilus, T.; Schackow, O.; Giannis, A. Chem. – Eur. J. 2017, 23, 5000–5004. doi:10.1002/chem.201701008 |
| 43. | Shirinian, V. Z.; Lvov, A. G.; Yadykov, A. V.; Yaminova, L. V.; Kachala, V. V.; Markosyan, A. I. Org. Lett. 2016, 18, 6260–6263. doi:10.1021/acs.orglett.6b03023 |
| 1. | Williams, D. R.; Robinson, L. A.; Nevill, C. R.; Reddy, J. P. Angew. Chem., Int. Ed. 2007, 46, 915–918. doi:10.1002/anie.200603853 |
| 2. | He, W.; Huang, J.; Sun, X.; Frontier, A. J. J. Am. Chem. Soc. 2008, 130, 300–308. doi:10.1021/ja0761986 |
| 3. | Wan, L.; Tius, M. A. Org. Lett. 2007, 9, 647–650. doi:10.1021/ol062919e |
| 4. | Harding, K. E.; Clement, K. S.; Tseng, C. Y. J. Org. Chem. 1990, 55, 4403–4410. doi:10.1021/jo00301a036 |
| 5. | Tius, M. A.; Drake, D. J. Tetrahedron 1996, 52, 14651–14660. doi:10.1016/0040-4020(96)00913-1 |
| 6. | Mateos, A. F.; Martín de la Nava, E. M.; González, R. R. J. Org. Chem. 2001, 66, 7632–7638. doi:10.1021/jo010449q |
| 7. | Teruya, T.; Nakagawa, S.; Koyama, T.; Arimoto, H.; Kita, M.; Uemura, D. Tetrahedron 2004, 60, 6989–6993. doi:10.1016/j.tet.2003.08.083 |
| 8. | Kang, H.-S.; Ji, S.-A.; Park, S.-H.; Kim, J.-P. Phytochemistry 2017, 143, 111–114. doi:10.1016/j.phytochem.2017.08.003 |
| 9. | Faiz, S.; Yousaf, M.; Zahoor, A. F.; Naqvi, S. A. R.; Irfan, A.; Zaman, G. Synth. Commun. 2017, 47, 1121–1135. doi:10.1080/00397911.2017.1303510 |
| 10. | Korabecny, J.; Spilovska, K.; Mezeiova, E.; Benek, O.; Juza, R.; Kaping, D.; Soukup, O. Curr. Med. Chem. 2019, 26, 5625–5648. doi:10.2174/0929867325666180517094023 |
| 11. | Patil, S. A.; Patil, R.; Patil, S. A. Eur. J. Med. Chem. 2017, 138, 182–198. doi:10.1016/j.ejmech.2017.06.032 |
| 12. | Li, M.; Xia, L. Chem. Biol. Drug Des. 2007, 70, 461–464. doi:10.1111/j.1747-0285.2007.00581.x |
| 32. | Liang, G.; Trauner, D. J. Am. Chem. Soc. 2004, 126, 9544–9545. doi:10.1021/ja0476664 |
| 75. | Kawatsura, M.; Higuchi, Y.; Hayase, S.; Nanjo, M.; Itoh, T. Synlett 2008, 1009–1012. doi:10.1055/s-2008-1072503 |
| 16. | Pellissier, H. Tetrahedron 2005, 61, 6479–6517. doi:10.1016/j.tet.2005.04.014 |
| 17. | Tius, M. A. Eur. J. Org. Chem. 2005, 2193–2206. doi:10.1002/ejoc.200500005 |
| 18. | Vinogradov, M. G.; Turova, O. V.; Zlotin, S. G. Org. Biomol. Chem. 2017, 15, 8245–8269. doi:10.1039/c7ob01981e |
| 19. | Wenz, D. R.; Read de Alaniz, J. Eur. J. Org. Chem. 2015, 23–37. doi:10.1002/ejoc.201402825 |
| 20. | Vaidya, T.; Eisenberg, R.; Frontier, A. J. ChemCatChem 2011, 3, 1531–1548. doi:10.1002/cctc.201100137 |
| 71. | Poisson, T.; Belhomme, M.-C.; Pannecoucke, X. J. Org. Chem. 2012, 77, 9277–9285. doi:10.1021/jo301873y |
| 72. | Chen, X.; Zhang, C.; Wu, H.; Yu, X.; Su, W.; Cheng, J. Synthesis 2007, 3233–3239. doi:10.1055/s-2007-990799 |
| 73. | Corey, E. J.; Suggs, J. W. Tetrahedron Lett. 1975, 16, 2647–2650. doi:10.1016/s0040-4039(00)75204-x |
| 74. | Davies, J.; Leonori, D. Chem. Commun. 2014, 50, 15171–15174. doi:10.1039/c4cc06501h |
| 16. | Pellissier, H. Tetrahedron 2005, 61, 6479–6517. doi:10.1016/j.tet.2005.04.014 |
| 17. | Tius, M. A. Eur. J. Org. Chem. 2005, 2193–2206. doi:10.1002/ejoc.200500005 |
| 18. | Vinogradov, M. G.; Turova, O. V.; Zlotin, S. G. Org. Biomol. Chem. 2017, 15, 8245–8269. doi:10.1039/c7ob01981e |
| 19. | Wenz, D. R.; Read de Alaniz, J. Eur. J. Org. Chem. 2015, 23–37. doi:10.1002/ejoc.201402825 |
| 20. | Vaidya, T.; Eisenberg, R.; Frontier, A. J. ChemCatChem 2011, 3, 1531–1548. doi:10.1002/cctc.201100137 |
| 24. | Dhoro, F.; Tius, M. A. J. Am. Chem. Soc. 2005, 127, 12472–12473. doi:10.1021/ja053393g |
| 71. | Poisson, T.; Belhomme, M.-C.; Pannecoucke, X. J. Org. Chem. 2012, 77, 9277–9285. doi:10.1021/jo301873y |
| 72. | Chen, X.; Zhang, C.; Wu, H.; Yu, X.; Su, W.; Cheng, J. Synthesis 2007, 3233–3239. doi:10.1055/s-2007-990799 |
| 73. | Corey, E. J.; Suggs, J. W. Tetrahedron Lett. 1975, 16, 2647–2650. doi:10.1016/s0040-4039(00)75204-x |
| 74. | Davies, J.; Leonori, D. Chem. Commun. 2014, 50, 15171–15174. doi:10.1039/c4cc06501h |
| 56. | Ollevier, T.; Lavie-Compin, G. Tetrahedron Lett. 2004, 45, 49–52. doi:10.1016/j.tetlet.2003.10.129 |
| 52. | Leonard, N. M.; Wieland, L. C.; Mohan, R. S. Tetrahedron 2002, 58, 8373–8397. doi:10.1016/s0040-4020(02)01000-1 |
| 53. | Bothwell, J. M.; Krabbe, S. W.; Mohan, R. S. Chem. Soc. Rev. 2011, 40, 4649–4707. doi:10.1039/c0cs00206b |
| 54. | Kakde, B. N.; Kumar, N.; Mondal, P. K.; Bisai, A. Org. Lett. 2016, 18, 1752–1755. doi:10.1021/acs.orglett.6b00446 |
| 11. | Patil, S. A.; Patil, R.; Patil, S. A. Eur. J. Med. Chem. 2017, 138, 182–198. doi:10.1016/j.ejmech.2017.06.032 |
| 80. | Lawrence, N. J.; Armitage, E. S. M.; Greedy, B.; Cook, D.; Ducki, S.; McGown, A. T. Tetrahedron Lett. 2006, 47, 1637–1640. doi:10.1016/j.tetlet.2005.12.110 |
| 81. | Prakasham, A. P.; Saxena, A. K.; Luqman, S.; Chanda, D.; Kaur, T.; Gupta, A.; Yadav, D. K.; Chanotiya, C. S.; Shanker, K.; Khan, F.; Negi, A. S. Bioorg. Med. Chem. 2012, 20, 3049–3057. doi:10.1016/j.bmc.2012.02.057 |
| 55. | Aggen, D. H.; Arnold, J. N.; Hayes, P. D.; Smoter, N. J.; Mohan, R. S. Tetrahedron 2004, 60, 3675–3679. doi:10.1016/j.tet.2004.02.046 |
| 82. | Bogeso, K. P.; Christensen, A. V.; Hyttel, J.; Liljefors, T. J. Med. Chem. 1985, 28, 1817–1828. doi:10.1021/jm00150a012 |
| 83. | Lee, B. H.; Choi, Y. L.; Shin, S.; Heo, J.-N. J. Org. Chem. 2011, 76, 6611–6618. doi:10.1021/jo2009164 |
| 84. | Yu, Y.-N.; Xu, M.-H. J. Org. Chem. 2013, 78, 2736–2741. doi:10.1021/jo302656s |
| 85. | Vicari, H. P.; Lima, K.; Gomes, R. d. C.; Fernandes, D. C.; da Silva, J. C. L.; Rodrigues Junior, M. T.; Barroso de Oliveira, A. S.; dos Santos, R. N.; Andricopulo, A. D.; Coelho, F.; Costa-Lotufo, L. V.; Machado-Neto, J. A. Eur. J. Pharmacol. 2021, 894, 173853. doi:10.1016/j.ejphar.2021.173853 |
| 64. | Prajapati, D.; Sandhu, J. S. Chem. Lett. 1992, 21, 1945–1946. doi:10.1246/cl.1992.1945 |
| 65. | Shen, Z.; Zhang, J.; Zou, H.; Yang, M. Tetrahedron Lett. 1997, 38, 2733–2736. doi:10.1016/s0040-4039(97)00456-5 |
| 62. | Cornélis, A.; Delaude, L.; Gerstmans, A.; Laszlo, P. Tetrahedron Lett. 1988, 29, 5657–5660. doi:10.1016/s0040-4039(00)80837-0 |
| 63. | Tanaka, H.; Nakahara, T.; Dhimane, H.; Torii, S. Tetrahedron Lett. 1989, 30, 4161–4164. doi:10.1016/s0040-4039(00)99348-1 |
| 61. | Samajdar, S.; Becker, F. F.; Banik, B. K. Synth. Commun. 2001, 31, 2691–2695. doi:10.1081/scc-100105397 |
| 57. | Leonard, N. M.; Oswald, M. C.; Freiberg, D. A.; Nattier, B. A.; Smith, R. C.; Mohan, R. S. J. Org. Chem. 2002, 67, 5202–5207. doi:10.1021/jo0258249 |
| 58. | Carrigan, M. D.; Sarapa, D.; Smith, R. C.; Wieland, L. C.; Mohan, R. S. J. Org. Chem. 2002, 67, 1027–1030. doi:10.1021/jo016180s |
| 59. | Ollevier, T.; Nadeau, E. J. Org. Chem. 2004, 69, 9292–9295. doi:10.1021/jo048617c |
© 2024 Rodrigues Jr. et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.