Abstract
A novel strategy for the synthesis of fusicoccane diterpenoids is reported. By harnessing the biosynthetic pathways of brassicicenes and fusicoccins, cotylenol was produced in an engineered Aspergillus oryzae strain. We further achieved the concise synthesis of three fusicoccane diterpenoids, including alterbrassicicene E and brassicicenes A and R in 4 or 5 chemical steps from brassicicene I. This strategy lays the foundation for the preparation of fusicoccane diterpenoids and their analogues for biological studies.
Graphical Abstract
Introduction
Fusicoccanes are a family of 5-8-5 tricyclic diterpenoid natural products that are produced by bacteria, fungi, algae, and plants (Figure 1a) [1-7]. Fusicoccanes possess a broad range of biological activities, including anticancer, anti-inflammatory, antimicrobial, antiparasitic, and plant growth regulating activities. For instance, cotylenin A (1) and fusicoccin A (2) function as molecular glues to stabilize the interactions between 14-3-3 proteins and their binding partners in plant and animal cells [8-12]. It has been reported that cotylenin A and its aglycone, cotylenol (3), induce differentiation in murine and human myeloid leukemia cells [13]. Cotylenin A and fusicoccin A also act synergistically with interferon-α or rapamycin to induce apoptosis in cancer cell lines [14-16]. However, cotylenin A cannot be produced by its natural source, Cladosporium sp. 501-7W, due to the loss of its ability to proliferate during preservation [17]. The important biological activities and complex structures of fusicoccane diterpenoids have inspired several total syntheses, which range between 15 and 29 steps [18-26]. Most of these synthetic approaches rely on similar strategies, i.e., coupling of the A ring and the C ring followed by the formation of the B ring. Additionally, the semisynthesis of analogues of 1 has been reported and led to the discovery of ISIR-050 (4), which shows higher activity than cotylenin A in cell growth inhibition assays and less toxicity in single-agent treatments [27,28]. Recently, Jiang and Renata described a chemoenzymatic approach that combines the skeletal construction by chemical methods and enzymatic C–H oxidations [29]. The synthesis employs a catalytic Nozaki–Hiyama–Kishi reaction and a one-pot Prins cyclization/transannular hydride transfer to construct the 5-8-5 tricyclic scaffold. Enzymatic oxidations were used to install the hydroxy group at the C-3 position. Ten fusicoccanes were synthesized in 8–13 steps each. Despite these efforts, a strategy with limited chemical transformations is highly desirable and should enable the discovery of new fusicoccane derivatives with improved biological activity.
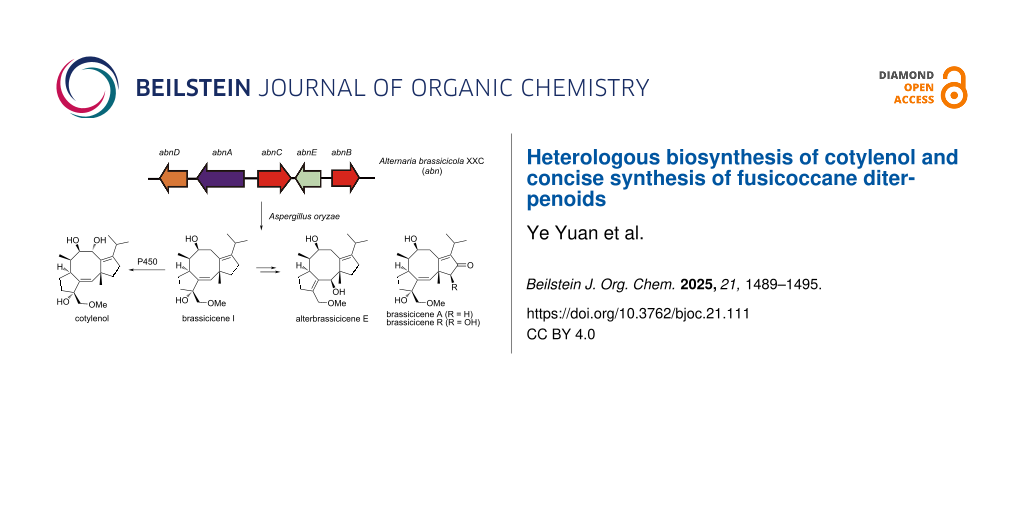
Figure 1: Selected fusicoccane diterpenoids and overview of this study. (a) Representative members of the fusicoccane diterpenoid family. (b) This work: heterologous biosynthesis of cotylenol (3) in an engineered Aspergillus oryzae strain and concise synthesis of fusicoccane diterpenoids.
Figure 1: Selected fusicoccane diterpenoids and overview of this study. (a) Representative members of the fus...
Inspired by the biosynthetic machinery of terpenoids, we have reported a hybrid synthetic strategy for accessing bioactive terpenoids by combining enzymatic terpene cyclization and chemical synthesis [30-33]. Briefly, the carbon scaffolds are forged by terpene cyclases, followed by concise chemical transformations to yield the desired natural products. Here, we describe heterologous biosynthesis of cotylenol by engineering the biosynthetic pathway of brassicicenes in Aspergillus oryzae and harnessing the promiscuity of a cytochrome P450 from the biosynthesis of fusicoccin A (Figure 1b). A key intermediate, brassicicenes I (5), was further used to achieve the collective synthesis of alterbrassicicene E (6), brassicicenes A (7) and R (8).
Results and Discussion
Fusicoccanes feature a characteristic dicyclopenta[a,d]cyclooctane (5-8-5) ring system that is biosynthesized from geranylgeranyl pyrophosphate (GGPP) via class I terpene cyclization (Figure 2a). To date, two fusicoccadiene synthases have been identified by the analysis of the brassicicene biosynthesis-related gene cluster (BGC) in Alternaria brassicicola and Pseudocercospora fijiensis [34,35]. The 5-8-5 tricyclic scaffold is transformed into various fusicoccane natural products catalyzed by P450s, dioxygenases, dehydrogenases, and reductases. Therefore, we propose to harness the biosynthetic pathway for brassicicenes, which share the same carbon skeleton and similar oxidation and unsaturation states as cotylenol and cotylenin A [36]. In a previous study, Oikawa and co-workers reported the identification of brassicicene BGC in Pseudocercospora fijiensis [37]. By heterologous expression of this BGC in Aspergillus oryzae, brassicicene I was produced by the transformant AO-bscABCDE at a titer of 5.5 mg/L. Recently, we identified a new BGC for brassicicenes, namely, abn, from the brassicicene-producing strain A. brassicicola XXC (Figure 2b) [38]. We constructed an A. oryzae strain with the homologous gene abnABCDE. As expected, compound 5 was produced at a titer of 8 mg/L (Figure 2c). By co-fermenting with Amberlite XAD-16, an enhanced yield of 30 mg/L was achieved, thus allowing further transformation into other natural products.
Figure 2: Heterologous production of brassicicene I in an engineered A. oryzae strain. (a) Biosynthesis of fusicoccin A in Phomopsis (Fusicoccum) amygdali. (b) Brassicicene BGC in A. brassicicola XXC. (c) Heterologous production of brassicicene I (5) in an engineered AO strain.
Figure 2: Heterologous production of brassicicene I in an engineered A. oryzae strain. (a) Biosynthesis of fu...
We next carried out the formal synthesis of cotylenin A and cotylenol (Figure 3a). Oxidation of brassicicene I with Dess–Martin reagent afforded intermediate 9 in 92% yield. The tertiary hydroxy group of compound 9 was further protected with a TMS group to provide compound 10 in 90% yield, a key intermediate in the synthesis of cotylenol and cotylenin A by Nakada and co-workers [21]. However, installing the C9 hydroxy group requires the use of stoichiometric MoOPH [39], which raises toxicity and safety issues. Therefore, we sought an enzymatic method to selectively oxidize 5 at the C9 position. Dairi and co-workers reported that Orf7 oxidizes compound 11 at the C9 position in the biosynthesis of fusicoccin A (Figure 3b) [40]. Given the structural similarities between compound 5 and compound 11, we hypothesized that Orf7 might also catalyze the hydroxylation of compound 5 at C9. Hence, we fed an A. oryzae strain that expressed the orf7 gene with compound 5. To our delight, compound 3 was obtained successfully (Figure 3c). To stably produce 3 by fermentation, we constructed an A. oryzae strain that integrates abnABCDE with orf7, achieving a yield of 60 mg/kg rice through rice fermentation.
Figure 3: Synthesis of cotylenol (3). (a) Synthesis of Nakada’s intermediate 10 from 5. (b) Orf7 catalyzes the oxidation of 11 in the biosynthesis of fusicoccin A (2). (c) LC–MS analysis of the production of 3 through AO-abnABCDE+orf7 heterologous expression or AO-orf7 biotransformation.
Figure 3: Synthesis of cotylenol (3). (a) Synthesis of Nakada’s intermediate 10 from 5. (b) Orf7 catalyzes th...
We next targeted alterbrassicicene E (6), brassicicenes A (7) and R (8) (Scheme 1). The secondary hydroxy group of brassicicene I was selectively TBS-protected in the presence of TBSOTf and 2,6-lutidine to give compound 13 in 93% yield. Then, compound 13 underwent oxidative rearrangement with PCC to afford ketone 14 in 61% yield. Under Luche reduction conditions, compound 15 and its diastereomer were obtained in a total yield of 90% at a ratio of 1:0.7. To improve the diastereoselectivity, we examined other reduction conditions and found that ʟ-Selectride afforded compound 15 in 90% yield with a dr of 9:1. Upon desilylation with TBAF, compound 15 was converted into alterbrassicicene E (6) in 80% yield. To synthesize brassicicenes A (7) and R (8), the tertiary hydroxy group of compound 13 was protected with a TES group to furnish compound 16 in 89% yield. By screening several conditions, we found that allylic oxidation of compound 16 could be achieved in the presence of chromium trioxide–3,5-dimethylpyrazole complex [41] to provide compound 17 in 76% yield. After deprotection of the TBS and TES groups with TBAF, brassicicene A (7) was obtained in 75% yield. Compound 17 was subjected to α-hydroxylation from the less-hindered convex face using Davis’s oxaziridine [25], furnishing intermediate 18 in 72% yield. After deprotection of the TBS and TES groups, brassicicene R (8) was obtained in 70% yield. Therefore, alterbrassicicene E (6) and brassicicenes A (7) and R (8) were synthesized from brassicicene I over 4 or 5 chemical steps.
Scheme 1: Synthesis of alterbrassicicene E (6) and brassicicenes A (7) and R (8) from brassicicene I (5).
Scheme 1: Synthesis of alterbrassicicene E (6) and brassicicenes A (7) and R (8) from brassicicene I (5).
Conclusion
In summary, the diverse biological activities and complex structures of fusicoccane diterpenoids have stimulated multiple elegant chemical syntheses. In contrast to these approaches, we harnessed the biosynthetic machinery of brassicicenes to produce brassicicene I in an engineered A. oryzae strain. Brassicicene I was further oxidized by a cytochrome P450 from the biosynthesis of fusicoccin A, thus leading to total biosynthesis of cotylenol in A. oryzae. Three fusicoccane diterpenoids, including alterbrassicicene E and brassicicenes A and R, were efficiently synthesized from brassicicene I in 4 or 5 chemical steps. This work lays the foundation for the preparation of fusicoccane natural products and exploration of their biological activities.
Supporting Information
| Supporting Information File 1: Experimental data and copies of spectra. | ||
| Format: PDF | Size: 3.2 MB | Download |
Acknowledgements
The authors thank Medical sub-center of analytical and testing center, Huazhong University of Science and Technology for measuring NMR spectroscopic data. The authors are very grateful to Professor Hideaki Oikawa (Hokkaido University, Sapporo, Japan) for providing A. oryzae NSAR1 heterologous expression system.
Funding
This work was financially supported by the National Key R&D Program of China (2021YFA0910500), the National Natural Science Foundation of China (22277035, 32000045, and 81973205), the Program for the National Natural Science Foundation for Distinguished Young Scholars (81725021), the Innovative Research Groups of the National Natural Science Foundation of China (81721005), the Fundamental Research Funds for the Central Universities (2020kfyXJJS083), the Sino-German Center for Research Promotion (M-0477), Key-Area Research and Development Program of Guangdong Province (2020B0303070002) and Shenzhen Science and Technology Program (KQTD20170330155106581).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
de Boer, A. H.; de Vries-van Leeuwen, I. J. Trends Plant Sci. 2012, 17, 360–368. doi:10.1016/j.tplants.2012.02.007
Return to citation in text: [1] -
Ballio, A.; Chain, E. B.; De Leo, P.; Erlanger, B. F.; Mauri, M.; Tonolo, A. Nature 1964, 203, 297. doi:10.1038/203297a0
Return to citation in text: [1] -
Sassa, T. Agric. Biol. Chem. 1971, 35, 1415–1418. doi:10.1271/bbb1961.35.1415
Return to citation in text: [1] -
Sassa, T.; Ooi, T.; Nukina, M.; Ikeda, M.; Kato, N. Biosci., Biotechnol., Biochem. 1998, 62, 1815–1818. doi:10.1271/bbb.62.1815
Return to citation in text: [1] -
Tang, Y.; Xue, Y.; Du, G.; Wang, J.; Liu, J.; Sun, B.; Li, X.-N.; Yao, G.; Luo, Z.; Zhang, Y. Angew. Chem., Int. Ed. 2016, 55, 4069–4073. doi:10.1002/anie.201600313
Return to citation in text: [1] -
Li, F.; Lin, S.; Zhang, S.; Pan, L.; Chai, C.; Su, J.-C.; Yang, B.; Liu, J.; Wang, J.; Hu, Z.; Zhang, Y. J. Nat. Prod. 2020, 83, 1931–1938. doi:10.1021/acs.jnatprod.0c00165
Return to citation in text: [1] -
Zhou, P.; Zhang, X.; Dai, C.; Yan, S.; Wei, M.; Feng, W.; Li, Q.; Liu, J.; Zhu, H.; Hu, Z.; Chen, C.; Zhang, Y. J. Org. Chem. 2022, 87, 7333–7341. doi:10.1021/acs.joc.2c00528
Return to citation in text: [1] -
Ohkanda, J. Chem. Lett. 2021, 50, 57–67. doi:10.1246/cl.200670
Return to citation in text: [1] -
Sengupta, A.; Liriano, J.; Bienkiewicz, E. A.; Miller, B. G.; Frederich, J. H. ACS Omega 2020, 5, 25029–25035. doi:10.1021/acsomega.0c01454
Return to citation in text: [1] -
Molzan, M.; Kasper, S.; Röglin, L.; Skwarczynska, M.; Sassa, T.; Inoue, T.; Breitenbuecher, F.; Ohkanda, J.; Kato, N.; Schuler, M.; Ottmann, C. ACS Chem. Biol. 2013, 8, 1869–1875. doi:10.1021/cb4003464
Return to citation in text: [1] -
Zheng, D.; Han, L.; Qu, X.; Chen, X.; Zhong, J.; Bi, X.; Liu, J.; Jiang, Y.; Jiang, C.; Huang, X. J. Nat. Prod. 2017, 80, 837–844. doi:10.1021/acs.jnatprod.6b00676
Return to citation in text: [1] -
Kim, S.; Shin, D.-S.; Lee, T.; Oh, K.-B. J. Nat. Prod. 2004, 67, 448–450. doi:10.1021/np030384h
Return to citation in text: [1] -
Asahi, K.-i.; Honma, Y.; Hazeki, K.; Sassa, T.; Kubohara, Y.; Sakurai, A.; Takahashi, N. Biochem. Biophys. Res. Commun. 1997, 238, 758–763. doi:10.1006/bbrc.1997.7385
Return to citation in text: [1] -
Honma, Y.; Kasukabe, T.; Yamori, T.; Kato, N.; Sassa, T. Gynecol. Oncol. 2005, 99, 680–688. doi:10.1016/j.ygyno.2005.07.015
Return to citation in text: [1] -
de Vries-van Leeuwen, I. J.; Kortekaas-Thijssen, C.; Nzigou Mandouckou, J. A.; Kas, S.; Evidente, A.; de Boer, A. H. Cancer Lett. 2010, 293, 198–206. doi:10.1016/j.canlet.2010.01.009
Return to citation in text: [1] -
Kasukabe, T.; Okabe‐Kado, J.; Honma, Y. Cancer Sci. 2008, 99, 1693–1698. doi:10.1111/j.1349-7006.2008.00867.x
Return to citation in text: [1] -
Ono, Y.; Minami, A.; Noike, M.; Higuchi, Y.; Toyomasu, T.; Sassa, T.; Kato, N.; Dairi, T. J. Am. Chem. Soc. 2011, 133, 2548–2555. doi:10.1021/ja107785u
Return to citation in text: [1] -
Liu, Y.; Hong, R. Cell Rep. Phys. Sci. 2024, 5, 102141. doi:10.1016/j.xcrp.2024.102141
Return to citation in text: [1] -
Kato, N.; Okamoto, H.; Takeshita, H. Tetrahedron 1996, 52, 3921–3932. doi:10.1016/s0040-4020(96)00059-2
Return to citation in text: [1] -
Williams, D. R.; Robinson, L. A.; Nevill, C. R.; Reddy, J. P. Angew. Chem., Int. Ed. 2007, 46, 915–918. doi:10.1002/anie.200603853
Return to citation in text: [1] -
Uwamori, M.; Osada, R.; Sugiyama, R.; Nagatani, K.; Nakada, M. J. Am. Chem. Soc. 2020, 142, 5556–5561. doi:10.1021/jacs.0c01774
Return to citation in text: [1] [2] -
Chen, B.; Wu, Q.; Xu, D.; Zhang, X.; Ding, Y.; Bao, S.; Zhang, X.; Wang, L.; Chen, Y. Angew. Chem., Int. Ed. 2022, 61, e202117476. doi:10.1002/anie.202117476
Return to citation in text: [1] -
Wang, Y.-Q.; Xu, K.; Min, L.; Li, C.-C. J. Am. Chem. Soc. 2022, 144, 10162–10167. doi:10.1021/jacs.2c04633
Return to citation in text: [1] -
Sims, N. J.; Bonnet, W. C.; Lawson, D. M.; Wood, J. L. J. Am. Chem. Soc. 2023, 145, 37–40. doi:10.1021/jacs.2c12275
Return to citation in text: [1] -
Chen, B.; Yang, Y.; Zhang, X.; Xu, D.; Sun, Y.; Chen, Y.; Wang, L. Org. Lett. 2023, 25, 8570–8574. doi:10.1021/acs.orglett.3c03355
Return to citation in text: [1] [2] -
Xie, S.; Chen, Y.; Zhang, Y.; Zhang, Z.; Hu, X.; Yan, C.; Xu, J. Cell Rep. Phys. Sci. 2024, 5, 101855. doi:10.1016/j.xcrp.2024.101855
Return to citation in text: [1] -
Inoue, T.; Higuchi, Y.; Yoneyama, T.; Lin, B.; Nunomura, K.; Honma, Y.; Kato, N. Bioorg. Med. Chem. Lett. 2018, 28, 646–650. doi:10.1016/j.bmcl.2018.01.030
Return to citation in text: [1] -
Ohkanda, J.; Kusumoto, A.; Punzalan, L.; Masuda, R.; Wang, C.; Parvatkar, P.; Akase, D.; Aida, M.; Uesugi, M.; Higuchi, Y.; Kato, N. Chem. – Eur. J. 2018, 24, 16066–16071. doi:10.1002/chem.201804428
Return to citation in text: [1] -
Jiang, Y.; Renata, H. Nat. Chem. 2024, 16, 1531–1538. doi:10.1038/s41557-024-01533-w
Return to citation in text: [1] -
You, Y.; Zhang, X.-J.; Xiao, W.; Kunthic, T.; Xiang, Z.; Xu, C. Chem. Sci. 2024, 15, 19307–19314. doi:10.1039/d4sc06060a
Return to citation in text: [1] -
Xiao, W.; Wang, S.-J.; Yu, M.-Z.; Zhang, X.-J.; Xiang, Z. Org. Biomol. Chem. 2023, 21, 5527–5531. doi:10.1039/d3ob00206c
Return to citation in text: [1] -
Mou, S.-B.; Xiao, W.; Wang, H.-Q.; Chen, K.-Y.; Xiang, Z. Org. Lett. 2021, 23, 400–404. doi:10.1021/acs.orglett.0c03894
Return to citation in text: [1] -
Mou, S.-B.; Xiao, W.; Wang, H.-Q.; Wang, S.-J.; Xiang, Z. Org. Lett. 2020, 22, 1976–1979. doi:10.1021/acs.orglett.0c00325
Return to citation in text: [1] -
Toyomasu, T.; Tsukahara, M.; Kaneko, A.; Niida, R.; Mitsuhashi, W.; Dairi, T.; Kato, N.; Sassa, T. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 3084–3088. doi:10.1073/pnas.0608426104
Return to citation in text: [1] -
Minami, A.; Tajima, N.; Higuchi, Y.; Toyomasu, T.; Sassa, T.; Kato, N.; Dairi, T. Bioorg. Med. Chem. Lett. 2009, 19, 870–874. doi:10.1016/j.bmcl.2008.11.108
Return to citation in text: [1] -
Guan, Z.; Yao, N.; Yuan, W.; Li, F.; Xiao, Y.; Rehmutulla, M.; Xie, Y.; Chen, C.; Zhu, H.; Zhou, Y.; Tong, Q.; Xiang, Z.; Ye, Y.; Zhang, Y. Chem. Sci. 2025, 16, 867–875. doi:10.1039/d4sc05963h
In parallel, we identified a biosynthetic pathway for cotylenol, cotylenins, from Talaromyces adpressus.
Return to citation in text: [1] -
Tazawa, A.; Ye, Y.; Ozaki, T.; Liu, C.; Ogasawara, Y.; Dairi, T.; Higuchi, Y.; Kato, N.; Gomi, K.; Minami, A.; Oikawa, H. Org. Lett. 2018, 20, 6178–6182. doi:10.1021/acs.orglett.8b02654
Return to citation in text: [1] -
Yuan, W.; Li, F.; Chen, Z.; Xu, Q.; Guan, Z.; Yao, N.; Hu, Z.; Liu, J.; Zhou, Y.; Ye, Y.; Zhang, Y. Chin. Chem. Lett. 2024, 35, 108788. doi:10.1016/j.cclet.2023.108788
Return to citation in text: [1] -
Vedejs, E.; Engler, D. A.; Telschow, J. E. J. Org. Chem. 1978, 43, 188–196. doi:10.1021/jo00396a002
Return to citation in text: [1] -
Noike, M.; Ono, Y.; Araki, Y.; Tanio, R.; Higuchi, Y.; Nitta, H.; Hamano, Y.; Toyomasu, T.; Sassa, T.; Kato, N.; Dairi, T. PLoS One 2012, 7, e42090. doi:10.1371/journal.pone.0042090
Return to citation in text: [1] -
Salmond, W. G.; Barta, M. A.; Havens, J. L. J. Org. Chem. 1978, 43, 2057–2059. doi:10.1021/jo00404a049
Return to citation in text: [1]
| 41. | Salmond, W. G.; Barta, M. A.; Havens, J. L. J. Org. Chem. 1978, 43, 2057–2059. doi:10.1021/jo00404a049 |
| 25. | Chen, B.; Yang, Y.; Zhang, X.; Xu, D.; Sun, Y.; Chen, Y.; Wang, L. Org. Lett. 2023, 25, 8570–8574. doi:10.1021/acs.orglett.3c03355 |
| 1. | de Boer, A. H.; de Vries-van Leeuwen, I. J. Trends Plant Sci. 2012, 17, 360–368. doi:10.1016/j.tplants.2012.02.007 |
| 2. | Ballio, A.; Chain, E. B.; De Leo, P.; Erlanger, B. F.; Mauri, M.; Tonolo, A. Nature 1964, 203, 297. doi:10.1038/203297a0 |
| 3. | Sassa, T. Agric. Biol. Chem. 1971, 35, 1415–1418. doi:10.1271/bbb1961.35.1415 |
| 4. | Sassa, T.; Ooi, T.; Nukina, M.; Ikeda, M.; Kato, N. Biosci., Biotechnol., Biochem. 1998, 62, 1815–1818. doi:10.1271/bbb.62.1815 |
| 5. | Tang, Y.; Xue, Y.; Du, G.; Wang, J.; Liu, J.; Sun, B.; Li, X.-N.; Yao, G.; Luo, Z.; Zhang, Y. Angew. Chem., Int. Ed. 2016, 55, 4069–4073. doi:10.1002/anie.201600313 |
| 6. | Li, F.; Lin, S.; Zhang, S.; Pan, L.; Chai, C.; Su, J.-C.; Yang, B.; Liu, J.; Wang, J.; Hu, Z.; Zhang, Y. J. Nat. Prod. 2020, 83, 1931–1938. doi:10.1021/acs.jnatprod.0c00165 |
| 7. | Zhou, P.; Zhang, X.; Dai, C.; Yan, S.; Wei, M.; Feng, W.; Li, Q.; Liu, J.; Zhu, H.; Hu, Z.; Chen, C.; Zhang, Y. J. Org. Chem. 2022, 87, 7333–7341. doi:10.1021/acs.joc.2c00528 |
| 17. | Ono, Y.; Minami, A.; Noike, M.; Higuchi, Y.; Toyomasu, T.; Sassa, T.; Kato, N.; Dairi, T. J. Am. Chem. Soc. 2011, 133, 2548–2555. doi:10.1021/ja107785u |
| 39. | Vedejs, E.; Engler, D. A.; Telschow, J. E. J. Org. Chem. 1978, 43, 188–196. doi:10.1021/jo00396a002 |
| 14. | Honma, Y.; Kasukabe, T.; Yamori, T.; Kato, N.; Sassa, T. Gynecol. Oncol. 2005, 99, 680–688. doi:10.1016/j.ygyno.2005.07.015 |
| 15. | de Vries-van Leeuwen, I. J.; Kortekaas-Thijssen, C.; Nzigou Mandouckou, J. A.; Kas, S.; Evidente, A.; de Boer, A. H. Cancer Lett. 2010, 293, 198–206. doi:10.1016/j.canlet.2010.01.009 |
| 16. | Kasukabe, T.; Okabe‐Kado, J.; Honma, Y. Cancer Sci. 2008, 99, 1693–1698. doi:10.1111/j.1349-7006.2008.00867.x |
| 40. | Noike, M.; Ono, Y.; Araki, Y.; Tanio, R.; Higuchi, Y.; Nitta, H.; Hamano, Y.; Toyomasu, T.; Sassa, T.; Kato, N.; Dairi, T. PLoS One 2012, 7, e42090. doi:10.1371/journal.pone.0042090 |
| 13. | Asahi, K.-i.; Honma, Y.; Hazeki, K.; Sassa, T.; Kubohara, Y.; Sakurai, A.; Takahashi, N. Biochem. Biophys. Res. Commun. 1997, 238, 758–763. doi:10.1006/bbrc.1997.7385 |
| 38. | Yuan, W.; Li, F.; Chen, Z.; Xu, Q.; Guan, Z.; Yao, N.; Hu, Z.; Liu, J.; Zhou, Y.; Ye, Y.; Zhang, Y. Chin. Chem. Lett. 2024, 35, 108788. doi:10.1016/j.cclet.2023.108788 |
| 8. | Ohkanda, J. Chem. Lett. 2021, 50, 57–67. doi:10.1246/cl.200670 |
| 9. | Sengupta, A.; Liriano, J.; Bienkiewicz, E. A.; Miller, B. G.; Frederich, J. H. ACS Omega 2020, 5, 25029–25035. doi:10.1021/acsomega.0c01454 |
| 10. | Molzan, M.; Kasper, S.; Röglin, L.; Skwarczynska, M.; Sassa, T.; Inoue, T.; Breitenbuecher, F.; Ohkanda, J.; Kato, N.; Schuler, M.; Ottmann, C. ACS Chem. Biol. 2013, 8, 1869–1875. doi:10.1021/cb4003464 |
| 11. | Zheng, D.; Han, L.; Qu, X.; Chen, X.; Zhong, J.; Bi, X.; Liu, J.; Jiang, Y.; Jiang, C.; Huang, X. J. Nat. Prod. 2017, 80, 837–844. doi:10.1021/acs.jnatprod.6b00676 |
| 12. | Kim, S.; Shin, D.-S.; Lee, T.; Oh, K.-B. J. Nat. Prod. 2004, 67, 448–450. doi:10.1021/np030384h |
| 21. | Uwamori, M.; Osada, R.; Sugiyama, R.; Nagatani, K.; Nakada, M. J. Am. Chem. Soc. 2020, 142, 5556–5561. doi:10.1021/jacs.0c01774 |
| 30. | You, Y.; Zhang, X.-J.; Xiao, W.; Kunthic, T.; Xiang, Z.; Xu, C. Chem. Sci. 2024, 15, 19307–19314. doi:10.1039/d4sc06060a |
| 31. | Xiao, W.; Wang, S.-J.; Yu, M.-Z.; Zhang, X.-J.; Xiang, Z. Org. Biomol. Chem. 2023, 21, 5527–5531. doi:10.1039/d3ob00206c |
| 32. | Mou, S.-B.; Xiao, W.; Wang, H.-Q.; Chen, K.-Y.; Xiang, Z. Org. Lett. 2021, 23, 400–404. doi:10.1021/acs.orglett.0c03894 |
| 33. | Mou, S.-B.; Xiao, W.; Wang, H.-Q.; Wang, S.-J.; Xiang, Z. Org. Lett. 2020, 22, 1976–1979. doi:10.1021/acs.orglett.0c00325 |
| 36. |
Guan, Z.; Yao, N.; Yuan, W.; Li, F.; Xiao, Y.; Rehmutulla, M.; Xie, Y.; Chen, C.; Zhu, H.; Zhou, Y.; Tong, Q.; Xiang, Z.; Ye, Y.; Zhang, Y. Chem. Sci. 2025, 16, 867–875. doi:10.1039/d4sc05963h
In parallel, we identified a biosynthetic pathway for cotylenol, cotylenins, from Talaromyces adpressus. |
| 29. | Jiang, Y.; Renata, H. Nat. Chem. 2024, 16, 1531–1538. doi:10.1038/s41557-024-01533-w |
| 37. | Tazawa, A.; Ye, Y.; Ozaki, T.; Liu, C.; Ogasawara, Y.; Dairi, T.; Higuchi, Y.; Kato, N.; Gomi, K.; Minami, A.; Oikawa, H. Org. Lett. 2018, 20, 6178–6182. doi:10.1021/acs.orglett.8b02654 |
| 27. | Inoue, T.; Higuchi, Y.; Yoneyama, T.; Lin, B.; Nunomura, K.; Honma, Y.; Kato, N. Bioorg. Med. Chem. Lett. 2018, 28, 646–650. doi:10.1016/j.bmcl.2018.01.030 |
| 28. | Ohkanda, J.; Kusumoto, A.; Punzalan, L.; Masuda, R.; Wang, C.; Parvatkar, P.; Akase, D.; Aida, M.; Uesugi, M.; Higuchi, Y.; Kato, N. Chem. – Eur. J. 2018, 24, 16066–16071. doi:10.1002/chem.201804428 |
| 18. | Liu, Y.; Hong, R. Cell Rep. Phys. Sci. 2024, 5, 102141. doi:10.1016/j.xcrp.2024.102141 |
| 19. | Kato, N.; Okamoto, H.; Takeshita, H. Tetrahedron 1996, 52, 3921–3932. doi:10.1016/s0040-4020(96)00059-2 |
| 20. | Williams, D. R.; Robinson, L. A.; Nevill, C. R.; Reddy, J. P. Angew. Chem., Int. Ed. 2007, 46, 915–918. doi:10.1002/anie.200603853 |
| 21. | Uwamori, M.; Osada, R.; Sugiyama, R.; Nagatani, K.; Nakada, M. J. Am. Chem. Soc. 2020, 142, 5556–5561. doi:10.1021/jacs.0c01774 |
| 22. | Chen, B.; Wu, Q.; Xu, D.; Zhang, X.; Ding, Y.; Bao, S.; Zhang, X.; Wang, L.; Chen, Y. Angew. Chem., Int. Ed. 2022, 61, e202117476. doi:10.1002/anie.202117476 |
| 23. | Wang, Y.-Q.; Xu, K.; Min, L.; Li, C.-C. J. Am. Chem. Soc. 2022, 144, 10162–10167. doi:10.1021/jacs.2c04633 |
| 24. | Sims, N. J.; Bonnet, W. C.; Lawson, D. M.; Wood, J. L. J. Am. Chem. Soc. 2023, 145, 37–40. doi:10.1021/jacs.2c12275 |
| 25. | Chen, B.; Yang, Y.; Zhang, X.; Xu, D.; Sun, Y.; Chen, Y.; Wang, L. Org. Lett. 2023, 25, 8570–8574. doi:10.1021/acs.orglett.3c03355 |
| 26. | Xie, S.; Chen, Y.; Zhang, Y.; Zhang, Z.; Hu, X.; Yan, C.; Xu, J. Cell Rep. Phys. Sci. 2024, 5, 101855. doi:10.1016/j.xcrp.2024.101855 |
| 34. | Toyomasu, T.; Tsukahara, M.; Kaneko, A.; Niida, R.; Mitsuhashi, W.; Dairi, T.; Kato, N.; Sassa, T. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 3084–3088. doi:10.1073/pnas.0608426104 |
| 35. | Minami, A.; Tajima, N.; Higuchi, Y.; Toyomasu, T.; Sassa, T.; Kato, N.; Dairi, T. Bioorg. Med. Chem. Lett. 2009, 19, 870–874. doi:10.1016/j.bmcl.2008.11.108 |
© 2025 Yuan et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.