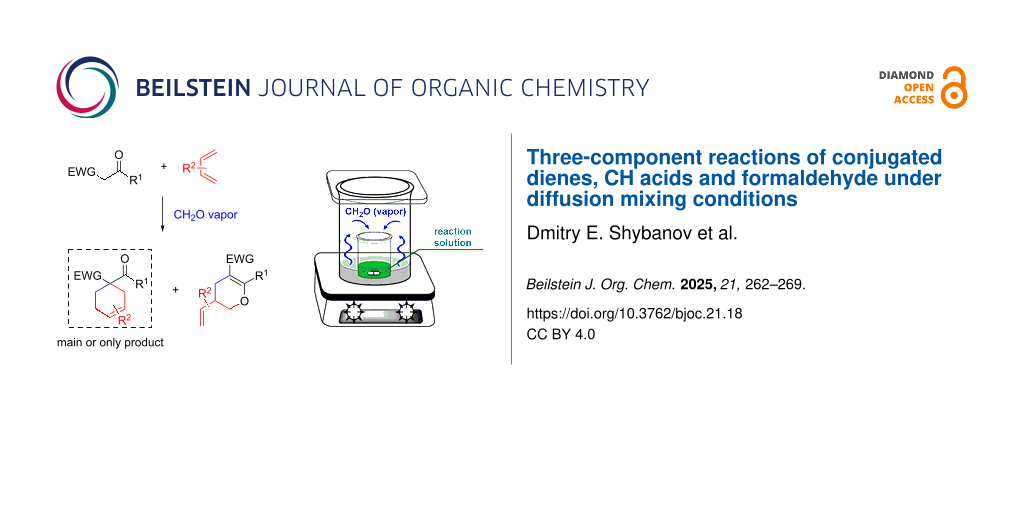
Abstract
A diffusion mixing technique with a volatile reagent was successfully used to generate crotonic condensation adducts of active methylene compounds and formaldehyde at room temperature in the absence of strong acids and bases. The formed adducts were highly reactive intermediates capable of reacting with dienes in a three-component reaction, leading to the formation of Diels–Alder main reaction products.
Graphical Abstract
Introduction
Formaldehyde is a reactive electrophilic reagent widely used as a C1 building block in multicomponent reactions [1-3]. Its role in most cases is to generate highly reactive species in situ from the nucleophilic reaction component. This can subsequently interact with other reaction components to form target products. Compared to the crotonic condensation products of other aliphatic and aromatic aldehydes, methylidene adducts of formaldehyde condensation are formed under milder conditions and are highly reactive, which is important for further synthetic transformations. However, due to the high carbonyl reactivity of formaldehyde, its interaction with active methylene compounds is often complicated by polycondensation [4-6] and polymerization processes of unstable methylidene adducts [7-9], instead of the desired formation of a monocrotonic product. Therefore, heating [6,10-14] or Lewis acid activation [15-18] have often been used in the literature for successful multicomponent reactions that proceed through the formation of formaldehyde condensation products (Scheme 1).
Scheme 1: Knoevenagel and Diels–Alder reactions in the multicomponent synthesis of substituted cyclohexadienes under formaldehyde participation.
Scheme 1: Knoevenagel and Diels–Alder reactions in the multicomponent synthesis of substituted cyclohexadiene...
Previously, we have proposed a convenient diffusion mixing technique for multicomponent reactions based on the absorption of volatile reagent vapors by a mixture containing the remaining reaction components. This method was successfully used to generate highly active nitrile oxides and nitrilimines for 1,3-dipolar cycloaddition reactions [19-21]. Based on our previous experience with diffusion mixing, we assumed that formaldehyde vapor diffusion into the reaction would lead to an extremely low concentration, which should significantly reduce undesirable polycondensations involving CH2O as well as polymerization of the intermediately formed methylidene derivatives. In this work, we generated methylidene adducts by formaldehyde condensation under diffusion mixing conditions in three-component reactions with various CH acid derivatives and conjugated dienes (cyclopentadiene, 1,3-cyclohexadiene, 2,3-dimethylbutadiene and isoprene), leading to [4 + 2]-cycloaddition adducts (Scheme 1).
It should be noted that in previous works describing three-component reactions of carbonyl compounds, conjugated dienes and formaldehyde (generated from paraformaldehyde upon heating), an adduct of a methylidene derivative of a CH acid dienophile was detected in some cases only in reference [15] (when carrying out the reaction in a sealed tube in the presence of copper(II) acetate). However, in most reactions only the hetero-Diels–Alder products were isolated from the reaction mixtures. In the present study, we carried out similar three-component reactions under significantly milder conditions (room temperature), and the main or only [4 + 2]-cycloaddition products in most cases were carbocyclic ones.
Results and Discussion
Interaction of 1,3-diketone 1 with formaldehyde
First, we investigated the ability of formaldehyde to interact with 1,3-diketone 1 using the diffusion mixing technique. For this purpose, we carried out a series of reactions in a very simple device consisting of a large vial with formalin, inside which we placed an open small vial with a solution of the other starting compounds (Figure 1). During the reaction, formaldehyde vapors from the outer vial were slowly absorbed by the mixture in the inner vial, which ensured an extremely low concentration of CH2O and intermediates in the reaction. An alternative to the diffusion mixing method is the slow dropwise addition of a reagent to the reaction mixture. However, in the latter case it is not possible to achieve uniform generation of active intermediates due to their formation in a local area, which accelerates unwanted side processes [19].
Figure 1: Equipment for carrying out reactions by the diffusion mixing method.
Figure 1: Equipment for carrying out reactions by the diffusion mixing method.
The results obtained are summarized in Scheme 2 and Table 1. It was found that the size of the vial used to carry out the reaction significantly affected the reaction rate. Thus, in an apparatus consisting of two vials with diameters of 1.3 and 2.8 cm, the conversion of the starting compound was 37 and 31%, respectively, when carrying out the reaction in chloroform or methanol over two days (Table 1, entries 3 and 7). In turn, in a system of two vials with diameters of 3.7 and 7.5 cm, and with a loading of diketone 1 that was five times greater, more than two thirds of the starting compound reacted within 24 h (Table 1, entries 11 and 13). Apparently, an increase in the surface area of the reaction solution promoted more efficient absorption of CH2O molecules, which accelerated the condensation reaction.
Scheme 2: Interaction of diketone 1 with formaldehyde under the diffusion mixing conditions.
Scheme 2: Interaction of diketone 1 with formaldehyde under the diffusion mixing conditions.
Table 1: Interaction of diketone 1 with formaldehyde under various conditions.
| entry | conditions | conversion of 1a [%] | yield of 2a [%] | yield of 3a [%] |
| 1 | petroleum ether, 2db | 57 | 16 | 30 |
| 2 | petroleum ether, 7db | 58 | 17 | 25 |
| 3 | CHCl3, 2db | 37 | 30 | trace |
| 4 | CHCl3, 7db | 77 | 48 | 21 |
| 5 | CDCl3, 2dc | 65 | 42 | 0 |
| 6 | Et2O, 2db | 13 | 8 | 0 |
| 7 | MeOH, 2db | 31 | 0 | 17 |
| 8 | EtOH, 2db | 68 | 0 | 64 |
| 9 | MeCN, 2db | 39 | 30 | trace |
| 10 | ʟ-proline (5 mol %), MeCN, 1db | 100 | 0 | 99 |
| 11 | CHCl3, 1dd | 82 | 48 | trace |
| 12 | CHCl3, 2dd | 96 | 27 | 31 |
| 13 | MeOH, 1dd | 69 | 29 | trace |
aBased on 1H NMR spectra of the reaction mixture. Compound 3 could be isolated in pure form, but compound 2 was unstable when isolated. bInner vial: diameter 1.3 cm, 0.09 mmol compound 1 in 5 mL solvent. Outer vial: diameter 2.8 cm, 3 mL of formalin. cInner vial: diameter 1.3 cm, 0.09 mmol compound 1 in 3 mL CDCl3. Outer vial: diameter 2.8 cm, 4 mL of formalin. After completion of the reaction, 550 μL of the solution were immediately placed in an ampule and analyzed by 1H NMR spectroscopy. dInner vial: diameter 3.7 cm, 0.45 mmol compound 1 in 10 mL solvent. Outer vial: diameter 7.5 cm, 6 mL of formalin.
The reaction of formaldehyde and diketone 1 in chloroform and acetonitrile predominantly led to diketo alcohol 2. Its 1H NMR spectrum contained a triplet with a coupling constant of 5.1 Hz at 5.52 ppm and a doublet at 4.30 ppm. In methanol, ethanol and petroleum ether, with an increasing reaction time, a significant amount of product 3 was observed in the 1H NMR spectra of the mixtures, as identified by characteristic triplets at 5.76 and 2.77 ppm (J = 7.0 Hz). Compound 3 was also formed in almost quantitative yield in acetonitrile within one day in the presence of ʟ-proline (Table 1, entry 10), which is an effective catalyst for crotonic condensation [22]. Since the reaction of diketone 1 in the presence and absence of ʟ-proline was carried out under otherwise identical conditions in the same apparatus (Table 1, entries 9 and 10), the significant difference in the conversion of compound 1 could not be explained only by the evaporation and absorption processes of CH2O. Apparently, the reaction rate also played an important role; the presence of the condensation catalyst ʟ-proline accelerated the absorption of formaldehyde vapors by the reaction mixture. Carrying out the same reaction in the absence of a catalyst in CDCl3 and subsequent 1H NMR spectroscopy analysis showed that the mixture contained 42% of keto alcohol 2 and 35% of diketone 1 (Table 1, entry 5), and only trace amounts of CH2O were present. This meant that formaldehyde introduction into the reaction mixture was very limited. Therefore, the condensation rate was the limiting step of the diffusion mixing.
The rapid formation of compound 3 in a high yield in the presence of ʟ-proline could be explained by the efficient crotonic condensation of formaldehyde and acetylacetone (1), followed by the addition of the second equivalent of diketone 1 to the highly reactive methylidene intermediate. The mild reaction conditions (room temperature) are worth mentioning since according to the literature, unstable crotonic condensation adducts are usually generated upon heating [6,10-13,15], in acidic medium [15,23] or using oxidizing agents [24,25].
Three-component reactions under diffusion mixing conditions
Under optimized conditions, we studied the possibility of generating active methylidene derivatives from malonic ester, Meldrum's acid, cyanoacetic acid ester, acetoacetic ester, acetylacetone and 1,3-diphenylpropane-1,3-dione (1). We found that, with the exception of malonic ester, all compounds reacted with formaldehyde under the above conditions to form highly reactive intermediates capable of [4 + 2]-cycloaddition reactions. Cyclopentadiene, cyclohexadiene, 2,3-dimethylbutadiene and isoprene were used as traps for the intermediates formed. The results are presented in Scheme 3. In general, the reactions produced adducts of the Diels–Alder (i.e., I) and the hetero-Diels–Alder reaction (i.e., II), or adducts resulting from the addition of a second equivalent of CH acid to the crotonic condensation product (i.e., III).
Scheme 3: Products of three-component reactions of methylene derivatives, formaldehyde and various dienes.
Scheme 3: Products of three-component reactions of methylene derivatives, formaldehyde and various dienes.
Apparently ʟ-proline played an essential role as catalyst in this three-component reaction. Using compounds 8 and 9, we could show that when the reaction was carried out in the absence of ʟ-proline, the conversion of the starting CH acid 1 after 5 days was less than 50%, and the main products present in the mixture were compounds 2 and 3. The proposed mechanism for the formation of compounds 8 and 9 with ʟ-proline participation is shown in Scheme 4. In the first step, formaldehyde reacts with proline, forming an imine salt 17, which then reacts with the diketone 1. The resulting intermediate 18 eliminates a proton and the anion of ʟ-proline, and then the methylenebenzophenone 20 reacts with cyclopentadiene to form the final products 8 and 9.
Scheme 4: Proposed mechanism for the formation of compounds 8 and 9 in the presence of ʟ-proline.
Scheme 4: Proposed mechanism for the formation of compounds 8 and 9 in the presence of ʟ-proline.
When using cyclopentadiene, it turned out that byproducts III were practically not formed, and the target compounds 4–6, 8 and 9 were isolated in a high overall yield. The moderate yield of compound 7 was due to the partial evaporation of the starting acetylacetone from the inner vial into the outer vessel containing formaldehyde, which affected the yield of all [4 + 2]-cycloaddition adducts involving this CH acid, regardless of the choice of diene. For acetoacetic ester and 1,3-diphenylpropane-1,3-dione, it was found that in addition to the main products 5 and 8, the reaction also produced hetero-Diels–Alder reaction adducts 6 and 9. For these, CH–O 1H NMR signals in the region of 4.9–5.4 ppm were characteristic.
It is worth noting that the individual isolated compounds 8 and particularly 9 were unstable when stored in solution, and boiling adduct 8 or 9 in toluene for 7 h led to the formation of an equilibrium mixture of these compounds in a ratio of ≈2:1 (Scheme 5).
Scheme 5: Interconversion of derivatives 8 and 9.
Scheme 5: Interconversion of derivatives 8 and 9.
We propose that the reversible transformation of 8 to 9 proceeded via the intermediate formation of zwitterion 21, in which the charges were stabilized by mesomeric effects under participation of the C=C bond. Apparently, the ratio of the adducts of the Diels–Alder (i.e., I) and the hetero-Diels–Alder reaction (i.e., II) was strongly influenced by steric factors; a decreased steric hindrance in the initial CH acid derivatives led to a more selective formation of the structures I. This was confirmed through the three-component reaction with acetylacetone, in which only the product 7 was formed (according to 1H NMR spectra of the reaction mixture).
Stereoselective interactions between methylidene adducts and cyclopentadiene were confirmed for three-component reactions involving cyanoacetic ester and acetylacetone. The configuration of the products 4 and 5 was established by bromination of a small amount of these isomeric compounds in CDCl3 (Scheme 6) and subsequent analysis of the mixture by 1H NMR spectroscopy. For the stereoisomers 4, the main product was lactone 22, identified by the signal of the CH–O group at 5.03 ppm, as well as by ethyl bromide, which indicated the predominance of isomer 4a in the mixture. For isomers 5, preferential formation of dibromide 23 and products of carbocationic rearrangements containing an ester group, the precursor of which was compound 5b, were found. The preferential formation of diastereomers 4a and 5b during the Diels–Alder reaction with the corresponding methylidene adducts was consistent with literature data [24,26].
Scheme 6: Interaction of 4a/4b and 5a/5b mixtures with bromine.
Scheme 6: Interaction of 4a/4b and 5a/5b mixtures with bromine.
Next, we studied three-component condensation reactions in the presence of the less active dienes cyclohexadiene, 2,3-dimethylbutadiene and isoprene. In reactions with Meldrum's acid, target products 10, 12 and 14 were obtained in a high yield. But for other CH acid derivatives, the reactions proceeded much less successful due to the side formation of products III. Under general conditions, the least active cyclohexadiene did not react even with acetylacetone, and for 2,3-dimethylbutadiene, target compound 13 was obtained in 41% yield. In all cases, no formation of even trace amounts of the hetero-Diels–Alder reaction adducts II was detected.
The regioselectivity of the cycloaddition of methylidene intermediates was also studied using isoprene as an example. In reactions involving Meldrum's acid, the formation of an inseparable mixture of the two stereoisomeric products 14a and 14b in a ratio of 95:5 was detected with a yield of 91%, the 1H NMR spectra of which coincided with those described in the literature [27]. The three-component reaction with acetoacetic ester was highly selective, and the formation of a minor [4 + 2]-cycloaddition product was not observed (according to 1H NMR spectroscopy), but the yield of the products 15 did not exceed 9%. For acetylacetone, the reaction was the least selective, resulting in an inseparable mixture of compounds 16a and 16b in a ratio of 87:13 and a total yield of 28%.
Conclusion
This work demonstrates that the technique of diffusion mixing with a volatile reagent can be successfully used to generate croton condensation adducts of active methylene compounds with formaldehyde at room temperature in the absence of strong acids and bases. These adducts are highly reactive intermediates capable of reacting with dienes in three-component reactions, leading to the formation of Diels–Alder main products or hetero-Diels–Alder adducts. In some cases, Michael addition products due to the addition of another equivalent of active methylene compound were also observed. Among the reactions studied, the diffusion mixing method gave the highest yield when using Meldrum’s acid as CH acid and in reactions with cyclopentadiene as diene.
Experimental
Materials and methods
All solvents used were purified and dehydrated using the methods described in reference [28]. All starting reagents were purchased from commercial sources (e.g., Sigma-Aldrich, abcr, AKSci). Reactions were checked by TLC analysis using silica plates with a fluorescent indicator (254 nm) and visualized with a UV lamp. 1H and 13C NMR spectra were recorded on Bruker Avance and Agilent 400-MR spectrometers (400 MHz for 1H, 100 MHz for 13C). Chemical shifts are reported in ppm relative to TMS.
General procedure for the three-component reactions under diffusion mixing conditions
A mixture of 1.0 mmol CH acid (1 equiv), 0.05 mmol ʟ-proline and 2.0–5.0 mmol diene (2–5 equiv) in 3–5 mL acetonitrile was placed into a 15 mL vial (diameter 1.3 cm). This vial was then placed into a closed 50 mL vial (diameter 2.8 cm) containing 3–5 mL of formalin, and the reaction mixture was stirred at room temperature for 2 days (TLC or NMR control). After completion of the reaction, the solvent was removed under reduced pressure, and the residue was purified by column chromatography on silica gel using chloroform as eluent.
((1S,4S)-Bicyclo[2.2.1]hept-5-ene-2,2-diyl)bis(phenylmethanone) (8) and phenyl((4aR,7aS)-2-phenyl-4,4a,5,7a-tetrahydrocyclopenta[b]pyran-3-yl)methanone (9). From 1,3-diphenylpropane-1,3-dione (224 mg, 1.0 mmol), ʟ-proline (6 mg, 0.05 mmol) and cyclopentadiene (330 mg, 5.0 mmol), compounds 8 (136 mg, 45%) and 9 (97 mg, 32%) were obtained as white crystalline solids.
Major isomer 8
1H NMR (400 MHz, CDCl3, δ) 7.98–7.89 (m, 4H), 7.45–7.38 (m, 2H), 7.36–7.28 (m, 4H), 6.30 (dd, J = 5.7, 3.0 Hz, 1H), 5.74 (dd, J = 5.7, 2.9 Hz, 1H), 3.94–3.90 (m, 1H), 3.00–2.95 (m, 1H), 2.84 (dd, J = 12.1, 2.9 Hz, 1H), 2.18 (dd, J = 12.1, 3.7 Hz, 1H), 1.76–1.71 (m, 1H), 1.63–1.54 (m, 1H); 13C NMR (101 MHz, CDCl3, δ) 200.0, 197.0, 140.1, 137.5, 136.6, 133.1, 133.0, 132.7, 129.9 (2C), 129.2 (2C), 128.6 (2C), 128.5 (2C), 71.9, 51.6, 49.3, 43.0, 36.9; HRMS–ESI+ (m/z): [M + H]+ calcd for C21H19O2, 303.1380; found, 303.1382.
Minor isomer 9
1H NMR (400 MHz, CDCl3, δ) 7.55–7.49 (m, 2H), 7.22–7.15 (m, 3H), 7.11–6.99 (m, 5H), 6.22–6.16 (m, 1H), 6.09–6.04 (m, 1H), 5.44–5.39 (m, 1H), 3.18–3.08 (m, 1H), 2.75 (dd, J = 14.3, 6.1 Hz, 1H), 2.71–2.62 (m, 1H), 2.58 (dd, J = 14.3, 4.8 Hz, 1H), 2.34–2.25 (m, 1H); 13C NMR (101 MHz, CDCl3, δ) 198.4, 165.1, 139.1, 137.5, 135.6, 131.4, 130.9, 129.7 (2C), 129.6 (2C), 129.5 (2C), 127.7 (3C), 115.0, 85.6, 39.3, 37.8, 27.4; HRMS–ESI+ (m/z): [M + H]+ calcd for C21H19O2, 303.1380; found, 303.1383.
Supporting Information
| Supporting Information File 1: 1H and 13C NMR spectra of synthesized compounds. | ||
| Format: PDF | Size: 2.5 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Li, W.; Wu, X.-F. Adv. Synth. Catal. 2015, 357, 3393–3418. doi:10.1002/adsc.201500753
Return to citation in text: [1] -
Liu, C.; Huang, W.; Zhang, J.; Rao, Z.; Gu, Y.; Jérôme, F. Green Chem. 2021, 23, 1447–1465. doi:10.1039/d0gc04124f
Return to citation in text: [1] -
Majumdar, K. C.; Taher, A.; Nandi, R. K. Tetrahedron 2012, 68, 5693–5718. doi:10.1016/j.tet.2012.04.098
Return to citation in text: [1] -
Bedenko, S. P.; Dement’ev, K. I.; Tret’yakov, V. F.; Maksimov, A. L. Pet. Chem. 2020, 60, 723–730. doi:10.1134/s0965544120070026
Return to citation in text: [1] -
Ogata, Y.; Kawasaki, A.; Yokoi, K. J. Chem. Soc. B 1967, 1013–1020. doi:10.1039/j29670001013
Return to citation in text: [1] -
Liu, C.; Shen, M.; Lai, B.; Taheri, A.; Gu, Y. ACS Comb. Sci. 2014, 16, 652–660. doi:10.1021/co5001019
Return to citation in text: [1] [2] [3] -
Burns, B. Rev. Adhes. Adhes. 2017, 5, 361–390. doi:10.7569/raa.2017.097309
Return to citation in text: [1] -
Guseva, T. I.; Senchenya, N. G.; Mager, K. A.; Tsyryapkin, V. A.; Gololobov, Y. G. Russ. Chem. Bull. 1994, 43, 595–598. doi:10.1007/bf00699831
Return to citation in text: [1] -
Bell, S. E. V.; Brown, R. F. C.; Eastwood, F. W.; Horvath, J. M. Aust. J. Chem. 2000, 53, 183–190. doi:10.1071/ch00016
Return to citation in text: [1] -
Gu, Y.; De Sousa, R.; Frapper, G.; Bachmann, C.; Barrault, J.; Jérôme, F. Green Chem. 2009, 11, 1968–1972. doi:10.1039/b913846c
Return to citation in text: [1] [2] -
Jiménez-Alonso, S.; Estévez-Braun, A.; Ravelo, Á. G.; Zárate, R.; López, M. Tetrahedron 2007, 63, 3066–3074. doi:10.1016/j.tet.2007.01.033
Return to citation in text: [1] [2] -
Nair, V.; Treesa, P. M. Tetrahedron Lett. 2001, 42, 4549–4551. doi:10.1016/s0040-4039(01)00741-9
Return to citation in text: [1] [2] -
Nair, V.; Jayan, C. N.; Radhakrishnan, K. V.; Anilkumar, G.; Rath, N. P. Tetrahedron 2001, 57, 5807–5813. doi:10.1016/s0040-4020(01)00471-9
Return to citation in text: [1] [2] -
Kukushkin, M.; Novotortsev, V.; Filatov, V.; Ivanenkov, Y.; Skvortsov, D.; Veselov, M.; Shafikov, R.; Moiseeva, A.; Zyk, N.; Majouga, A.; Beloglazkina, E. Molecules 2021, 26, 7645. doi:10.3390/molecules26247645
Return to citation in text: [1] -
Nenajdenko, V. G.; Statsuk, A. V.; Balenkova, E. S. Tetrahedron 2000, 56, 6549–6556. doi:10.1016/s0040-4020(00)00605-0
Return to citation in text: [1] [2] [3] [4] -
Epifano, F.; Pelucchini, C.; Rosati, O.; Genovese, S.; Curini, M. Catal. Lett. 2011, 141, 844–849. doi:10.1007/s10562-011-0605-3
Return to citation in text: [1] -
Akhmadiev, N. S.; Akhmetova, V. R.; Boiko, T. F.; Ibragimov, A. G. Chem. Heterocycl. Compd. 2018, 54, 344–350. doi:10.1007/s10593-018-2271-5
Return to citation in text: [1] -
Kumar, A.; Sharma, S.; Maurya, R. A. Tetrahedron Lett. 2009, 50, 5937–5940. doi:10.1016/j.tetlet.2009.08.046
Return to citation in text: [1] -
Shybanov, D. E.; Filkina, M. E.; Kukushkin, M. E.; Grishin, Y. K.; Roznyatovsky, V. A.; Zyk, N. V.; Beloglazkina, E. K. New J. Chem. 2022, 46, 18575–18586. doi:10.1039/d2nj03756d
Return to citation in text: [1] [2] -
Shybanov, D. E.; Kukushkin, M. E.; Hrytseniuk, Y. S.; Grishin, Y. K.; Roznyatovsky, V. A.; Tafeenko, V. A.; Skvortsov, D. A.; Zyk, N. V.; Beloglazkina, E. K. Int. J. Mol. Sci. 2023, 24, 5037. doi:10.3390/ijms24055037
Return to citation in text: [1] -
Kuznetsova, J. V.; Tkachenko, V. T.; Petrovskaya, L. M.; Filkina, M. E.; Shybanov, D. E.; Grishin, Y. K.; Roznyatovsky, V. A.; Tafeenko, V. A.; Pestretsova, A. S.; Yakovleva, V. A.; Pokrovsky, V. S.; Kukushkin, M. E.; Beloglazkina, E. K. Int. J. Mol. Sci. 2024, 25, 18. doi:10.3390/ijms25010018
Return to citation in text: [1] -
Karmakar, R.; Mukhopadhyay, C. Tetrahedron Chem 2024, 11, 100087. doi:10.1016/j.tchem.2024.100087
Return to citation in text: [1] -
Zia-Ebrahimi, M.; Huffman, G. W. Synthesis 1996, 215–218. doi:10.1055/s-1996-4201
Return to citation in text: [1] -
Hoye, T. R.; Caruso, A. J.; Magee, A. S. J. Org. Chem. 1982, 47, 4152–4156. doi:10.1021/jo00142a028
Return to citation in text: [1] [2] -
Eberle, M.; Lawton, R. G. Helv. Chim. Acta 1988, 71, 1974–1982. doi:10.1002/hlca.19880710816
Return to citation in text: [1] -
Mellor, J. M.; Webb, C. F. J. Chem. Soc., Perkin Trans. 2 1974, 17–22. doi:10.1039/p29740000017
Return to citation in text: [1] -
Burke, D. J.; Kawauchi, T.; Kade, M. J.; Leibfarth, F. A.; McDearmon, B.; Wolffs, M.; Kierstead, P. H.; Moon, B.; Hawker, C. J. ACS Macro Lett. 2012, 1, 1228–1232. doi:10.1021/mz300497m
Return to citation in text: [1] -
Tietze, L. F.; Eicher, T. Reaktionen und Synthesen im organisch-chemischen Praktikum und Forschungslaboratorium, 2nd ed.; Wiley-VCH: Weinheim, Germany, 1991. doi:10.1002/3527601716
Return to citation in text: [1]
| 1. | Li, W.; Wu, X.-F. Adv. Synth. Catal. 2015, 357, 3393–3418. doi:10.1002/adsc.201500753 |
| 2. | Liu, C.; Huang, W.; Zhang, J.; Rao, Z.; Gu, Y.; Jérôme, F. Green Chem. 2021, 23, 1447–1465. doi:10.1039/d0gc04124f |
| 3. | Majumdar, K. C.; Taher, A.; Nandi, R. K. Tetrahedron 2012, 68, 5693–5718. doi:10.1016/j.tet.2012.04.098 |
| 15. | Nenajdenko, V. G.; Statsuk, A. V.; Balenkova, E. S. Tetrahedron 2000, 56, 6549–6556. doi:10.1016/s0040-4020(00)00605-0 |
| 16. | Epifano, F.; Pelucchini, C.; Rosati, O.; Genovese, S.; Curini, M. Catal. Lett. 2011, 141, 844–849. doi:10.1007/s10562-011-0605-3 |
| 17. | Akhmadiev, N. S.; Akhmetova, V. R.; Boiko, T. F.; Ibragimov, A. G. Chem. Heterocycl. Compd. 2018, 54, 344–350. doi:10.1007/s10593-018-2271-5 |
| 18. | Kumar, A.; Sharma, S.; Maurya, R. A. Tetrahedron Lett. 2009, 50, 5937–5940. doi:10.1016/j.tetlet.2009.08.046 |
| 28. | Tietze, L. F.; Eicher, T. Reaktionen und Synthesen im organisch-chemischen Praktikum und Forschungslaboratorium, 2nd ed.; Wiley-VCH: Weinheim, Germany, 1991. doi:10.1002/3527601716 |
| 6. | Liu, C.; Shen, M.; Lai, B.; Taheri, A.; Gu, Y. ACS Comb. Sci. 2014, 16, 652–660. doi:10.1021/co5001019 |
| 10. | Gu, Y.; De Sousa, R.; Frapper, G.; Bachmann, C.; Barrault, J.; Jérôme, F. Green Chem. 2009, 11, 1968–1972. doi:10.1039/b913846c |
| 11. | Jiménez-Alonso, S.; Estévez-Braun, A.; Ravelo, Á. G.; Zárate, R.; López, M. Tetrahedron 2007, 63, 3066–3074. doi:10.1016/j.tet.2007.01.033 |
| 12. | Nair, V.; Treesa, P. M. Tetrahedron Lett. 2001, 42, 4549–4551. doi:10.1016/s0040-4039(01)00741-9 |
| 13. | Nair, V.; Jayan, C. N.; Radhakrishnan, K. V.; Anilkumar, G.; Rath, N. P. Tetrahedron 2001, 57, 5807–5813. doi:10.1016/s0040-4020(01)00471-9 |
| 14. | Kukushkin, M.; Novotortsev, V.; Filatov, V.; Ivanenkov, Y.; Skvortsov, D.; Veselov, M.; Shafikov, R.; Moiseeva, A.; Zyk, N.; Majouga, A.; Beloglazkina, E. Molecules 2021, 26, 7645. doi:10.3390/molecules26247645 |
| 7. | Burns, B. Rev. Adhes. Adhes. 2017, 5, 361–390. doi:10.7569/raa.2017.097309 |
| 8. | Guseva, T. I.; Senchenya, N. G.; Mager, K. A.; Tsyryapkin, V. A.; Gololobov, Y. G. Russ. Chem. Bull. 1994, 43, 595–598. doi:10.1007/bf00699831 |
| 9. | Bell, S. E. V.; Brown, R. F. C.; Eastwood, F. W.; Horvath, J. M. Aust. J. Chem. 2000, 53, 183–190. doi:10.1071/ch00016 |
| 24. | Hoye, T. R.; Caruso, A. J.; Magee, A. S. J. Org. Chem. 1982, 47, 4152–4156. doi:10.1021/jo00142a028 |
| 26. | Mellor, J. M.; Webb, C. F. J. Chem. Soc., Perkin Trans. 2 1974, 17–22. doi:10.1039/p29740000017 |
| 4. | Bedenko, S. P.; Dement’ev, K. I.; Tret’yakov, V. F.; Maksimov, A. L. Pet. Chem. 2020, 60, 723–730. doi:10.1134/s0965544120070026 |
| 5. | Ogata, Y.; Kawasaki, A.; Yokoi, K. J. Chem. Soc. B 1967, 1013–1020. doi:10.1039/j29670001013 |
| 6. | Liu, C.; Shen, M.; Lai, B.; Taheri, A.; Gu, Y. ACS Comb. Sci. 2014, 16, 652–660. doi:10.1021/co5001019 |
| 27. | Burke, D. J.; Kawauchi, T.; Kade, M. J.; Leibfarth, F. A.; McDearmon, B.; Wolffs, M.; Kierstead, P. H.; Moon, B.; Hawker, C. J. ACS Macro Lett. 2012, 1, 1228–1232. doi:10.1021/mz300497m |
| 22. | Karmakar, R.; Mukhopadhyay, C. Tetrahedron Chem 2024, 11, 100087. doi:10.1016/j.tchem.2024.100087 |
| 15. | Nenajdenko, V. G.; Statsuk, A. V.; Balenkova, E. S. Tetrahedron 2000, 56, 6549–6556. doi:10.1016/s0040-4020(00)00605-0 |
| 23. | Zia-Ebrahimi, M.; Huffman, G. W. Synthesis 1996, 215–218. doi:10.1055/s-1996-4201 |
| 19. | Shybanov, D. E.; Filkina, M. E.; Kukushkin, M. E.; Grishin, Y. K.; Roznyatovsky, V. A.; Zyk, N. V.; Beloglazkina, E. K. New J. Chem. 2022, 46, 18575–18586. doi:10.1039/d2nj03756d |
| 24. | Hoye, T. R.; Caruso, A. J.; Magee, A. S. J. Org. Chem. 1982, 47, 4152–4156. doi:10.1021/jo00142a028 |
| 25. | Eberle, M.; Lawton, R. G. Helv. Chim. Acta 1988, 71, 1974–1982. doi:10.1002/hlca.19880710816 |
| 15. | Nenajdenko, V. G.; Statsuk, A. V.; Balenkova, E. S. Tetrahedron 2000, 56, 6549–6556. doi:10.1016/s0040-4020(00)00605-0 |
| 19. | Shybanov, D. E.; Filkina, M. E.; Kukushkin, M. E.; Grishin, Y. K.; Roznyatovsky, V. A.; Zyk, N. V.; Beloglazkina, E. K. New J. Chem. 2022, 46, 18575–18586. doi:10.1039/d2nj03756d |
| 20. | Shybanov, D. E.; Kukushkin, M. E.; Hrytseniuk, Y. S.; Grishin, Y. K.; Roznyatovsky, V. A.; Tafeenko, V. A.; Skvortsov, D. A.; Zyk, N. V.; Beloglazkina, E. K. Int. J. Mol. Sci. 2023, 24, 5037. doi:10.3390/ijms24055037 |
| 21. | Kuznetsova, J. V.; Tkachenko, V. T.; Petrovskaya, L. M.; Filkina, M. E.; Shybanov, D. E.; Grishin, Y. K.; Roznyatovsky, V. A.; Tafeenko, V. A.; Pestretsova, A. S.; Yakovleva, V. A.; Pokrovsky, V. S.; Kukushkin, M. E.; Beloglazkina, E. K. Int. J. Mol. Sci. 2024, 25, 18. doi:10.3390/ijms25010018 |
| 6. | Liu, C.; Shen, M.; Lai, B.; Taheri, A.; Gu, Y. ACS Comb. Sci. 2014, 16, 652–660. doi:10.1021/co5001019 |
| 10. | Gu, Y.; De Sousa, R.; Frapper, G.; Bachmann, C.; Barrault, J.; Jérôme, F. Green Chem. 2009, 11, 1968–1972. doi:10.1039/b913846c |
| 11. | Jiménez-Alonso, S.; Estévez-Braun, A.; Ravelo, Á. G.; Zárate, R.; López, M. Tetrahedron 2007, 63, 3066–3074. doi:10.1016/j.tet.2007.01.033 |
| 12. | Nair, V.; Treesa, P. M. Tetrahedron Lett. 2001, 42, 4549–4551. doi:10.1016/s0040-4039(01)00741-9 |
| 13. | Nair, V.; Jayan, C. N.; Radhakrishnan, K. V.; Anilkumar, G.; Rath, N. P. Tetrahedron 2001, 57, 5807–5813. doi:10.1016/s0040-4020(01)00471-9 |
| 15. | Nenajdenko, V. G.; Statsuk, A. V.; Balenkova, E. S. Tetrahedron 2000, 56, 6549–6556. doi:10.1016/s0040-4020(00)00605-0 |
© 2025 Shybanov et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.