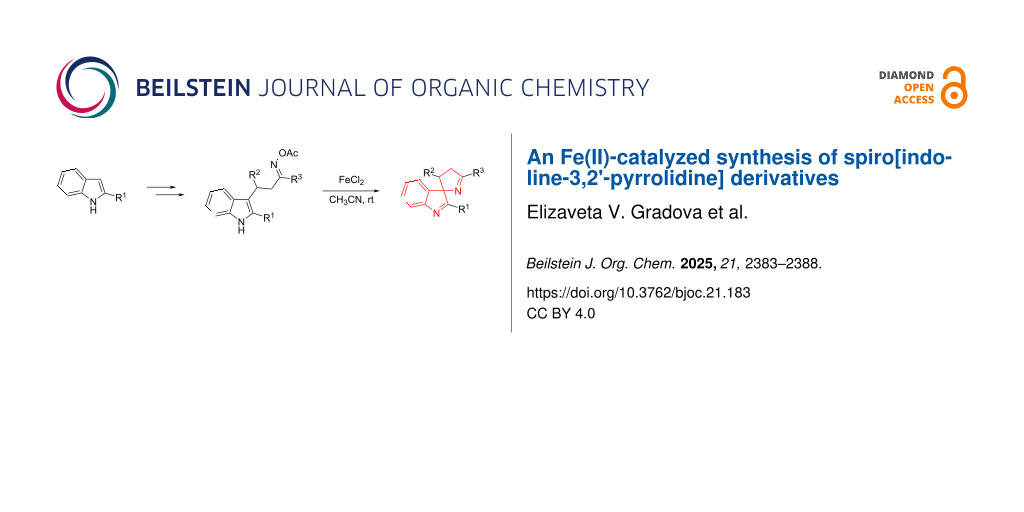
Abstract
A synthetic strategy for the preparation of spiro[indoline-3,2'-pyrrolidine] derivatives has been developed, featuring a two-step sequence consisting of the reaction of 2-arylindoles with α,β-unsaturated ketones, followed by Fe(II)-catalyzed spirocyclization of readily accessible oxime acetates. The method exhibits a broad substrate scope and good functional group tolerance. The synthesized spirocyclic compounds showed no significant antimicrobial activity.
Graphical Abstract
Introduction
Spiro[indoline-3,2'-pyrrolidine] derivatives represent an important class of organic compounds found in both natural products (e.g., coerulescine [1], horsfiline [2], and elacomine [3]) and synthetic bioactive molecules (Figure 1). This scaffold exhibits a broad range of pharmacological activities, including significant in vitro antimycobacterial properties [4], potent antitumor effects against melanoma cell lines [5], and antagonism of TH2 lymphocyte function [6]. Due to their wide-ranging biological activities, spiro[indoline-3,2'-pyrrolidines] have attracted substantial interest in medicinal chemistry, prompting the development of diverse synthetic strategies.
Figure 1: Natural and synthetic bioactive spiro[indoline-3,2'-pyrrolidine] derivatives.
Figure 1: Natural and synthetic bioactive spiro[indoline-3,2'-pyrrolidine] derivatives.
Several methodologies have been reported for the synthesis of substituted spiro[indoline-3,2'-pyrrolidines] (Scheme 1), which can be broadly categorized into two main approaches. The first involves cascade reactions featuring the formation of multiple bonds, including a pivotal spirocyclization step. However, these strategies typically require pre-functionalized starting materials and often result exclusively in substituted oxindoles. For example, a highly diastereoselective method for the synthesis of dihydrospiro[indoline-3,2'-pyrrole]-2-ones has been developed (Scheme 1, path a) [7]. This transformation proceeds via a Lewis acid-mediated conjugate addition of vinyl azides to electron-deficient alkenes, followed by denitrogenative cyclization. Subsequently Zhong et al. reported a catalytic asymmetric variant, affording spirooxindoles in high yields with excellent enantioselectivity [8]. An alternative approach employing vinyl azides involves a Rh(II)-catalyzed olefination of diazo compounds, followed by annulation with vinyl azides to yield substituted spiropyrrolidines (Scheme 1, path b) [9]. Additionally, an organocatalytic, enantioselective Michael addition/cyclization sequence of 3-aminooxindole Schiff bases with terminal vinyl ketones, catalyzed by a cinchona-derived base, has been reported to afford chiral spiroindolylpyrroles in high yields (Scheme 1, path c) [10]. Further expanding the scope of enantioselective approaches, a Michael/cyclization cascade reaction between 3-aminooxindoles and 2-enoylpyridines, catalyzed by a cinchonidine-based thiourea organocatalyst, was developed. Subsequent treatment with HCl in methanol under heating furnished chiral spiroindolylpyrroles in excellent yields and enantioselectivity (Scheme 1, path d) [11]. Moreover, a copper-catalyzed reaction of oxindole-derived alkenes with acetophenone O-acetyl oxime has also been employed to construct the spiroindolylpyrrole scaffold (Scheme 1, path e) [12].
Scheme 1: Previous approaches and our work.
Scheme 1: Previous approaches and our work.
The second category of synthetic methods relies on more accessible, non-pre-functionalized starting materials and stepwise assembly of the spirocyclic core. This strategy enables the synthesis of functionalized 3H-indoles, which can be further elaborated into structurally diverse products. For instance, an intramolecular SN2-type cyclization of β-3-indolyl ketone oximes using MsCl and Et₃N affords spiro[indoline-3,2'-pyrrolidines] (Scheme 1, path f) [13]. A subsequent Cu-catalyzed spirocyclization of β-3-indolyl ketone oxime acetates was developed. This process involves homolytic N–O bond cleavage to generate an N-imidoyl radical intermediate that undergoes intramolecular cyclization to yield the spirocyclic product (Scheme 1, path g) [14]. Notably, iron is known to exhibit similar behavior in single-electron transfer (SET) processes [15-17]. In fact, we previously demonstrated an Fe-catalyzed dearomatization of β-2-furyl ketone oxime acetates, yielding functionalized pyrroles [18]. Herein, we report our investigation of the Fe-catalyzed spirocyclization of β-3-indolyl ketone oxime acetates.
Results and Discussion
Our study commenced with the synthesis of key propan-1-one derivatives bearing an indolyl moiety. For this purpose, the reaction of 2-arylindoles 1 with α,β-unsaturated ketones 2 was employed, affording the corresponding indolylalkanones 3 (Scheme 2) [19-21]. A combination of trimethylsilyl chloride and acetonitrile served as a mild promoter for the desired reaction [17]. Under these conditions, the reaction was performed on a 5 mmol scale without any loss of efficiency. We next evaluated the scope of the developed protocol. The electronic and steric nature of substituents had a minimal impact on the product yields and in most cases, the desired products were obtained in good to high yields. However, two notable exceptions were identified. First, when a nitro group was introduced at the para-position of the benzoyl moiety the starting chalcone was recovered unchanged. Second, the use of an α,β-unsaturated ketone bearing an N-methylpyrrole moiety resulted in extensive decomposition and tarring, a common issue with α-unsubstituted pyrroles under acidic conditions. These results indicate that substrates featuring strongly electron-withdrawing groups or acid-sensitive motifs are not compatible with the developed protocol.
Scheme 2: The reaction of 2-arylindoles 1 with α,β-unsaturated ketones 2. aIsolated yield of the 5 mmol scale experiment.
Scheme 2: The reaction of 2-arylindoles 1 with α,β-unsaturated ketones 2. aIsolated yield of the 5 mmol scale...
Next, we synthesized the model ketone oxime acetate from β-3-indolyl ketone 3a using the previously described telescopic protocol [17]. This substrate was subjected to FeCl2-catalyzed spirocyclization in acetonitrile at room temperature, affording the desired spirocyclic product 4a in 70% yield as a mixture of diastereomers (dr = 1:10). Scaling up the reaction to a 4 mmol scale led to a decrease in yield to 55%. Under these conditions, we explored the substrate scope of the reaction (Scheme 3). In general, substituent effects were minimal, and most spirocyclic products were obtained in good to high yields. Nevertheless, several notable exceptions were observed. First, when a substrate bearing an alkyl substituent at the keto group was employed, the yield of the desired product 4o decreased to 20%. Second, the use of a 2-naphthylindole substrate (3l) afforded no desired product, presumably due to increased steric demand. Third, the introduction of an ortho-methyl substituent on the ketone moiety (3m) likewise suppressed product formation, likely due to steric hindrance interfering with cyclization at the C3 position of the indole ring.
Scheme 3: The scope of the Fe-catalyzed spirocyclization. aIsolated yield of the 4.2 mmol scale experiment.
Scheme 3: The scope of the Fe-catalyzed spirocyclization. aIsolated yield of the 4.2 mmol scale experiment.
Based on literature precedents [15,16], we propose a mechanism involving a radical pathway (Scheme 4). Initial Fe(II)-mediated reductive cleavage of the N–O bond in the ketoxime acetate generates an iminyl radical. This is followed by a 5-exo-trig cyclization to form a carbon-centered radical. Final single-electron oxidation by Fe(III) delivers the desired spirocyclic product.
Scheme 4: The proposed mechanism of product 4 formation.
Scheme 4: The proposed mechanism of product 4 formation.
All synthesized spiro[indoline-3,2'-pyrrolidine] derivatives 4 were evaluated for antimicrobial activity via serial dilution assays across a concentration range of 0.5–1000 µg/mL. The minimum inhibitory concentration (MIC) and minimum bactericidal (fungicidal) concentration (MBC/MFC) against a diverse spectrum of microorganisms was determined, including two fungal strains: Candida albicans ATCC 10231, C. albicans C1 – clinical strain, Gram-positive bacteria such as Staphylococcus aureus ATCC 25923, S. aureus ATCC 43300 (MRSA), Mycobacterium smegmatis ATCC 70084, and Gram-negative bacteria including Escherichia coli ATCC 25922, E. coli ATCC 8739, and Klebsiella pneumoniae ATCC 700603. Notably, none of the tested compounds demonstrated significant antibacterial activity against the evaluated strains (for details, see Supporting Information File 1).
Conclusion
In summary, we have developed an efficient synthetic strategy for constructing spiro[indoline-3,2'-pyrrolidine] derivatives via a sequence involving the reaction of 2-arylindoles with α,β-unsaturated ketones, followed by Fe(II)-catalyzed spirocyclization of the corresponding easily accessible oxime acetates. The methodology exhibits broad substrate scope, with only minor limitations attributable to steric hindrance or functional group sensitivity. Antimicrobial evaluation of the synthesized spirocyclic compounds revealed no significant activity against the tested microbial strains. However, the synthetic versatility and broad substrate scope of developed protocol highlights its potential for further derivatization and the discovery of valuable properties.
Supporting Information
| Supporting Information File 1: General reaction procedures, compound characterization data, and copies of NMR spectra. | ||
| Format: PDF | Size: 5.7 MB | Download |
Acknowledgements
We are grateful to the associate professor of Microbiology and Virology Dept., leading researcher of Central Research Laboratory of E. A. Vagner Perm State Medical University (Perm, Russian Federation) Godovalov A. P. for providing the clinical strains.
Funding
We acknowledge the Russian Science Foundation (grant no. 24-73-10099, https://rscf.ru/en/project/24-73-10099/) for financial support. Spectral studies were conducted with the support of the Ministry of Science and Higher Education of the Russian Federation (Contract No. 0750-2025-0010).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Cossins, E. A.; Chen, L. Phytochemistry 1997, 45, 437–452. doi:10.1016/s0031-9422(96)00833-3
Return to citation in text: [1] -
Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527–6530. doi:10.1021/jo00023a016
Return to citation in text: [1] -
Pellegrini, C.; Weber, M.; Borschberg, H.-J. Helv. Chim. Acta 1996, 79, 151–168. doi:10.1002/hlca.19960790116
Return to citation in text: [1] -
Maheswari, S. U.; Balamurugan, K.; Perumal, S.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2010, 20, 7278–7282. doi:10.1016/j.bmcl.2010.10.080
Return to citation in text: [1] -
Girgis, A. S.; Panda, S. S.; Srour, A. M.; Farag, H.; Ismail, N. S. M.; Elgendy, M.; Abdel-Aziz, A. K.; Katritzky, A. R. Org. Biomol. Chem. 2015, 13, 6619–6633. doi:10.1039/c5ob00410a
Return to citation in text: [1] -
Crosignani, S.; Page, P.; Missotten, M.; Colovray, V.; Cleva, C.; Arrighi, J.-F.; Atherall, J.; Macritchie, J.; Martin, T.; Humbert, Y.; Gaudet, M.; Pupowicz, D.; Maio, M.; Pittet, P.-A.; Golzio, L.; Giachetti, C.; Rocha, C.; Bernardinelli, G.; Filinchuk, Y.; Scheer, A.; Schwarz, M. K.; Chollet, A. J. Med. Chem. 2008, 51, 2227–2243. doi:10.1021/jm701383e
Return to citation in text: [1] -
Zhu, X.; Chiba, S. Chem. Commun. 2016, 52, 2473–2476. doi:10.1039/c5cc10299e
Return to citation in text: [1] -
Zhong, Z.; Xiao, Z.; Liu, X.; Cao, W.; Feng, X. Chem. Sci. 2020, 11, 11492–11497. doi:10.1039/d0sc03776a
Return to citation in text: [1] -
Yi, R.; Qian, L.; Wan, B. Chin. J. Catal. 2019, 40, 177–183. doi:10.1016/s1872-2067(18)63200-0
Return to citation in text: [1] -
Huang, Z.-C.; Zou, Y.; Xiang, M.; Li, C.-Y.; Li, X.; Tian, F.; Wang, L.-X. Org. Lett. 2021, 23, 2227–2231. doi:10.1021/acs.orglett.1c00370
Return to citation in text: [1] -
Cui, B.; Chen, Y.; Shan, J.; Qin, L.; Yuan, C.; Wang, Y.; Han, W.; Wan, N.; Chen, Y. Org. Biomol. Chem. 2017, 15, 8518–8522. doi:10.1039/c7ob02138k
Return to citation in text: [1] -
Zhao, B.; Liang, H.-W.; Yang, J.; Yang, Z.; Wei, Y. ACS Catal. 2017, 7, 5612–5617. doi:10.1021/acscatal.7b01876
Return to citation in text: [1] -
Tanaka, K.; Mori, Y.; Narasaka, K. Chem. Lett. 2004, 33, 26–27. doi:10.1246/cl.2004.26
Return to citation in text: [1] -
Wang, P.-F.; Chen, C.; Chen, H.; Han, L.-S.; Liu, L.; Sun, H.; Wen, X.; Xu, Q.-L. Adv. Synth. Catal. 2017, 359, 2339–2344. doi:10.1002/adsc.201700073
Return to citation in text: [1] -
Zhu, Z.; Tang, X.; Li, J.; Li, X.; Wu, W.; Deng, G.; Jiang, H. Org. Lett. 2017, 19, 1370–1373. doi:10.1021/acs.orglett.7b00203
Return to citation in text: [1] [2] -
Wu, C.; Liu, T.-X.; Zhang, P.; Zhu, X.; Zhang, G. Org. Lett. 2020, 22, 7327–7332. doi:10.1021/acs.orglett.0c02658
Return to citation in text: [1] [2] -
Liang, W.; Jiang, K.; Du, F.; Yang, J.; Shuai, L.; Ouyang, Q.; Chen, Y.-C.; Wei, Y. Angew. Chem., Int. Ed. 2020, 59, 19222–19228. doi:10.1002/anie.202007825
Return to citation in text: [1] [2] [3] -
Makarov, A. S.; Fadeev, A. A.; Uchuskin, M. G. Org. Chem. Front. 2021, 8, 6553–6560. doi:10.1039/d1qo01281a
Return to citation in text: [1] -
Fadeev, A. A.; Uchuskin, M. G.; Trushkov, I. V.; Makarov, A. S. Chem. Heterocycl. Compd. 2017, 53, 1286–1293. doi:10.1007/s10593-018-2206-1
Return to citation in text: [1] -
Liu, J.; Zhang, Y.; Yue, Y.; Wang, Z.; Shao, H.; Zhuo, K.; Lv, Q.; Zhang, Z. J. Org. Chem. 2019, 84, 12946–12959. doi:10.1021/acs.joc.9b01586
Return to citation in text: [1] -
Patel, T.; Gaikwad, R.; Jain, K.; Ganesh, R.; Bobde, Y.; Ghosh, B.; Das, K.; Gayen, S. ChemistrySelect 2019, 4, 4478–4482. doi:10.1002/slct.201900088
Return to citation in text: [1]
| 17. | Liang, W.; Jiang, K.; Du, F.; Yang, J.; Shuai, L.; Ouyang, Q.; Chen, Y.-C.; Wei, Y. Angew. Chem., Int. Ed. 2020, 59, 19222–19228. doi:10.1002/anie.202007825 |
| 19. | Fadeev, A. A.; Uchuskin, M. G.; Trushkov, I. V.; Makarov, A. S. Chem. Heterocycl. Compd. 2017, 53, 1286–1293. doi:10.1007/s10593-018-2206-1 |
| 20. | Liu, J.; Zhang, Y.; Yue, Y.; Wang, Z.; Shao, H.; Zhuo, K.; Lv, Q.; Zhang, Z. J. Org. Chem. 2019, 84, 12946–12959. doi:10.1021/acs.joc.9b01586 |
| 21. | Patel, T.; Gaikwad, R.; Jain, K.; Ganesh, R.; Bobde, Y.; Ghosh, B.; Das, K.; Gayen, S. ChemistrySelect 2019, 4, 4478–4482. doi:10.1002/slct.201900088 |
| 17. | Liang, W.; Jiang, K.; Du, F.; Yang, J.; Shuai, L.; Ouyang, Q.; Chen, Y.-C.; Wei, Y. Angew. Chem., Int. Ed. 2020, 59, 19222–19228. doi:10.1002/anie.202007825 |
| 1. | Cossins, E. A.; Chen, L. Phytochemistry 1997, 45, 437–452. doi:10.1016/s0031-9422(96)00833-3 |
| 5. | Girgis, A. S.; Panda, S. S.; Srour, A. M.; Farag, H.; Ismail, N. S. M.; Elgendy, M.; Abdel-Aziz, A. K.; Katritzky, A. R. Org. Biomol. Chem. 2015, 13, 6619–6633. doi:10.1039/c5ob00410a |
| 15. | Zhu, Z.; Tang, X.; Li, J.; Li, X.; Wu, W.; Deng, G.; Jiang, H. Org. Lett. 2017, 19, 1370–1373. doi:10.1021/acs.orglett.7b00203 |
| 16. | Wu, C.; Liu, T.-X.; Zhang, P.; Zhu, X.; Zhang, G. Org. Lett. 2020, 22, 7327–7332. doi:10.1021/acs.orglett.0c02658 |
| 17. | Liang, W.; Jiang, K.; Du, F.; Yang, J.; Shuai, L.; Ouyang, Q.; Chen, Y.-C.; Wei, Y. Angew. Chem., Int. Ed. 2020, 59, 19222–19228. doi:10.1002/anie.202007825 |
| 4. | Maheswari, S. U.; Balamurugan, K.; Perumal, S.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2010, 20, 7278–7282. doi:10.1016/j.bmcl.2010.10.080 |
| 18. | Makarov, A. S.; Fadeev, A. A.; Uchuskin, M. G. Org. Chem. Front. 2021, 8, 6553–6560. doi:10.1039/d1qo01281a |
| 3. | Pellegrini, C.; Weber, M.; Borschberg, H.-J. Helv. Chim. Acta 1996, 79, 151–168. doi:10.1002/hlca.19960790116 |
| 13. | Tanaka, K.; Mori, Y.; Narasaka, K. Chem. Lett. 2004, 33, 26–27. doi:10.1246/cl.2004.26 |
| 2. | Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527–6530. doi:10.1021/jo00023a016 |
| 14. | Wang, P.-F.; Chen, C.; Chen, H.; Han, L.-S.; Liu, L.; Sun, H.; Wen, X.; Xu, Q.-L. Adv. Synth. Catal. 2017, 359, 2339–2344. doi:10.1002/adsc.201700073 |
| 9. | Yi, R.; Qian, L.; Wan, B. Chin. J. Catal. 2019, 40, 177–183. doi:10.1016/s1872-2067(18)63200-0 |
| 11. | Cui, B.; Chen, Y.; Shan, J.; Qin, L.; Yuan, C.; Wang, Y.; Han, W.; Wan, N.; Chen, Y. Org. Biomol. Chem. 2017, 15, 8518–8522. doi:10.1039/c7ob02138k |
| 8. | Zhong, Z.; Xiao, Z.; Liu, X.; Cao, W.; Feng, X. Chem. Sci. 2020, 11, 11492–11497. doi:10.1039/d0sc03776a |
| 12. | Zhao, B.; Liang, H.-W.; Yang, J.; Yang, Z.; Wei, Y. ACS Catal. 2017, 7, 5612–5617. doi:10.1021/acscatal.7b01876 |
| 15. | Zhu, Z.; Tang, X.; Li, J.; Li, X.; Wu, W.; Deng, G.; Jiang, H. Org. Lett. 2017, 19, 1370–1373. doi:10.1021/acs.orglett.7b00203 |
| 16. | Wu, C.; Liu, T.-X.; Zhang, P.; Zhu, X.; Zhang, G. Org. Lett. 2020, 22, 7327–7332. doi:10.1021/acs.orglett.0c02658 |
| 6. | Crosignani, S.; Page, P.; Missotten, M.; Colovray, V.; Cleva, C.; Arrighi, J.-F.; Atherall, J.; Macritchie, J.; Martin, T.; Humbert, Y.; Gaudet, M.; Pupowicz, D.; Maio, M.; Pittet, P.-A.; Golzio, L.; Giachetti, C.; Rocha, C.; Bernardinelli, G.; Filinchuk, Y.; Scheer, A.; Schwarz, M. K.; Chollet, A. J. Med. Chem. 2008, 51, 2227–2243. doi:10.1021/jm701383e |
| 10. | Huang, Z.-C.; Zou, Y.; Xiang, M.; Li, C.-Y.; Li, X.; Tian, F.; Wang, L.-X. Org. Lett. 2021, 23, 2227–2231. doi:10.1021/acs.orglett.1c00370 |
© 2025 Gradova et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.