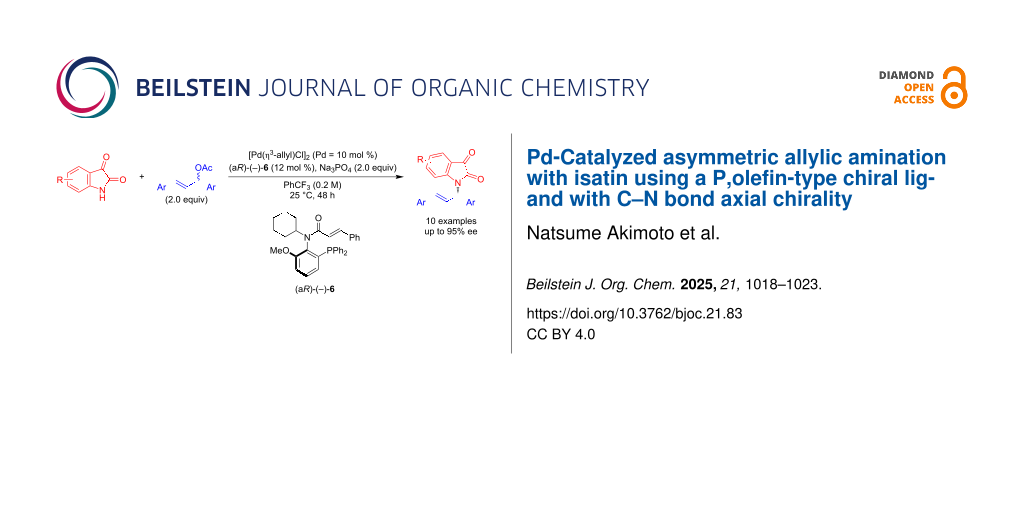
Abstract
In this study, we implemented the P,olefin-type chiral ligand (aR)-(−)-6, which contains a cyclohexyl group and a cinnamoyl group on the nitrogen atom, in the Pd-catalyzed asymmetric allylic amination of allylic esters with isatin derivatives 11 as nucleophiles. The reaction proceeds efficiently, yielding the products (S)-13 with good-to-high enantioselectivity. A scale-up reaction was also successfully conducted at a 1 mmol scale. Additionally, when malononitrile was added to the resulting product (S)-13a in the presence of FeCl3 as the catalyst, the corresponding malononitrile derivative (S)-16 was obtained without any loss in optical purity.
Graphical Abstract
Introduction
Isatin is a well-known natural indole derivative. Due to the broad biological activities of its derivatives, extensive research has been conducted on their synthesis. Furthermore, the isatin framework is a versatile starting material for various transformations, including multicomponent reactions and the synthesis of spirocyclic compounds [1-3]. The nucleophilicity of isatin at the nitrogen atom allows it to participate in reactions such as alkylation [4], arylation [5], and aza-Michael addition [6-8]. However, the products obtained from these reactions are primarily achiral or racemic, and only a few studies have reported the use of isatin as a nucleophile in asymmetric reactions [9-11]. On the other hand, it has been revealed that compounds in which the carbon bonded to the nitrogen atom of newly constructed N-substituted isatin becomes a chiral center exhibit pharmacological properties in medicinal chemistry. For example, racemic compound 1 (Figure 1) was evaluated for its cytotoxicity against human breast cancer cells (MCF7) in comparison to the standard doxorubicin and exhibited excellent activity against the MCF7 cell line [12]. The optically active compound 2 also showed activity against Huh7.5-FGR-JC1-Rluc2A cells, which carry HCV gt 2a [13].
Therefore, developing asymmetric reactions that simultaneously form a carbon–nitrogen bond and construct a chiral center is of great importance. Although a relatively large number of asymmetric allylic amination reactions using palladium catalysts with amines as nucleophiles have been reported [14-25], there have been only a few reports on the N-substitution of isatin using asymmetric methods. Recently, Wolf’s group reported a transition-metal-catalyzed (Pd-catalyzed) asymmetric allylic amination of allyl esters using isatin as a nucleophile. In this reaction, bisphosphine-type ligands such as BINAP and SEGPHOS derivatives, as well as P,N-type ligands like oxazoline-type ligands, were utilized as chiral ligands [26]. On the other hand, several groups have recently reported new chiral ligands with axial chirality for Pd-catalyzed asymmetric allylic substitution reactions. For example, the Zhou group reported a P,olefin-type chiral ligand 3 with C–C bond axial chirality for this reaction (Figure 2) [27]. Additionally, we have recently reported chiral ligands with C–N bond axial chirality, such as N-alkyl-N-cinnamyl-type chiral ligands 4 [28,29] and 5 [30], and a P,olefin-type chiral ligand 6 [31] with a cinnamoyl group instead of a cinnamyl group. In particular, the chiral ligand 6 is effective in the Pd-catalyzed asymmetric allylic substitution reaction of allylic esters with indoles. Here, we describe the Pd-catalyzed asymmetric allylic amination of allylic esters with isatin as a nucleophile using chiral ligand 6 and its derivative 7. Compared to chiral ligand 6, which has a secondary alkyl group (cyclohexyl) as a substituent on the nitrogen and has already been reported, compound 7 has a primary alkyl group (n-propyl). This difference reduces steric hindrance and lowers the rotational barrier around the carbon–nitrogen bond, increasing the likelihood of racemization.
Results and Discussion
N-Propyl-N-cinnamoylamide 7 was prepared from phosphine oxide 8 [32] via an SNAr reaction with nucleophilic lithium amide from n-propylamine, the reduction of phosphine oxide 9 by trichlorosilane/triethylamine, and the N-acylation of 10 with cinnamoyl chloride in three steps (Scheme 1). We also analyzed amide compound 7 by HPLC analysis using a chiral stationary phase column with a CD detector and found that the C(aryl)–N(amide) bond axial chirality exists in amide compound 7. We attempted the optical resolution of racemic compound (±)-7 and obtained (+)-7 and (−)-7 using a semi-preparative chiral HPLC on 50 milligram scales. We also investigated the racemization process associated with the axial chirality of compound 7 (see Supporting Information File 1). The racemization barrier (ΔG‡rac) of (−)-7 in n-dodecane was determined to be 25.0 kcal/mol at 25 °C, as calculated using the Arrhenius and Eyring equations [33-35]. Therefore, the half-life of racemization of ligand (−)-7 at 25 °C in n-dodecane is approximately 1.3 days, which is faster compared to ligand 6, which has a half-life of about 3.7 days [31].
Scheme 1: Preparation and optical resolution of 7.
Scheme 1: Preparation and optical resolution of 7.
We next investigated the ability of optically active amides (aR)-(−)-6 and (−)-7 as chiral ligands for the Pd-catalyzed asymmetric allylic amination of allylic acetate, such as a 1,3-diphenyl-2-propenyl acetate (12) with isatin (11a). We began the investigation under conditions using 5 mol % of [Pd(C3H5)Cl]2 (Pd = 10 mol %) and 12 mol % of chiral ligands (Table 1).
Table 1: Optimization of conditions for the Pd-catalyzed asymmetric allylic amination of acetate 12 with isatin (11a).a
|
|
||||
| Entry | Base | Solvent | Yield (%)b | ee (%)c |
| 1 | K2CO3 | CHCl3 | 72 | 87 |
| 2d | K2CO3 | CHCl3 | 3 | 84 |
| 3 | Na2CO3 | CHCl3 | 99 | 85 |
| 4 | Cs2CO3 | CHCl3 | 19 | 86 |
| 5 | NaOAc | CHCl3 | 89 | 86 |
| 6 | K3PO4 | CHCl3 | 12 | 86 |
| 7 | Na3PO4 | CHCl3 | 60 | 88 |
| 8 | Na3PO4 | CH2Cl2 | 88 | 92 |
| 9 | Na3PO4 | CH3CN | 75 | 93 |
| 10 | Na3PO4 | THF | 74 | 93 |
| 11 | Na3PO4 | DMF | trace | – |
| 12 | Na3PO4 | PhCF3 | 84 | 95 |
| 13e | Na3PO4 | PhCF3 | 50 | 86 |
| 14f | Na3PO4 | PhCF3 | 80 | 94 |
aThe reaction was carried out at 0.1 mmol scale. bIsolated yield. cDetermined by chiral HPLC analysis using a chiral column. Absolute configuration was assigned by comparison of HPLC analysis with reported data [26]. dThis reaction was carried out using (−)-7 instead of (aR)-(−)-6 as a chiral ligand. eThis reaction was carried out using 1,3-diphenylallyl pivalate (14) instead of acetate 12. fThis reaction was carried out at a 1.0 mmol scale.
The reaction with (aR)-(−)-6 as the chiral ligand and K2CO3 as the base in CHCl3 gave the desired product (S)-13a in 72% yield with 87% ee (Table 1, entry 1). In contrast, the reaction with (−)-7 afforded (S)-13a in significantly lower yield, albeit with an enantioselectivity similar to that of the reaction with 6 (Table 1, entry 2). This result clarifies that (−)-7, with a racemization half-life of only approximately 1.3 days, also has a chiral induction ability. However, improvement is required in terms of the reactivity of the catalytic reaction. Subsequently, we investigated the effect of the base using (aR)-(−)-6 by testing various bases. The reaction in the presence of Na2CO3 delivered the product in 99% yield, although the enantioselectivity slightly decreased compared to the reaction using K2CO3 (see Table 1, entry 1 vs entry 3). The use of Cs2CO3 resulted in a significant drop in the yield (Table 1, entry 4), whereas NaOAc improved the yield but slightly lowered the enantioselectivity (Table 1, entry 5). Other potassium salts such as K3PO4 led to a low yield of the product (Table 1, entry 6). Meanwhile, when Na3PO4 was tested, the yield decreased, but the enantioselectivity improved to 88% ee (Table 1, entry 7). With Na3PO4 as the optimum base, which showed the highest enantioselectivity, we conducted a solvent screening. The reaction in CH2Cl2 resulted in better yield and enantioselectivity than in CHCl3 (Table 1, entry 8). The coordinating solvents, CH3CN and THF, further improved the enantioselectivity to 93% ee (Table 1, entries 9 and 10). In contrast, the reaction barely proceeded when DMF was used (Table 1, entry 11). The reaction in PhCF3 afforded the target product in a good yield with the highest enantioselectivity compared to other solvents (Table 1, entry 12). Furthermore, when (E)-1,3-diphenyl-2-propenyl pivalate (14) was tested as the allyl ester, the desired product (S)-13a was obtained with a yield of 50% and an enantioselectivity of 86% ee (Table 1, entry 13). Additionally, the scale-up reaction using 1 mmol of isatin (11a) as the nucleophile under the optimal conditions (Table 1, entry 12) afforded the desired product (S)-13a with nearly the same yield and enantioselectivity as the 0.1 mmol scale reaction (entry 14).
Next, we investigated the substrate scope of the palladium-catalyzed asymmetric allylic amination of 1,3-diphenyl-2-propenyl acetate (12) with isatin derivatives 11 as nucleophiles under the optimized conditions using (aR)-(−)-6 as the ligand and Na3PO4 as the base in PhCF3 as the solvent (Scheme 2). An isatin derivative bearing a chloro group at the 4-position afforded the desired product (S)-13b with good yield and enantioselectivity. Similarly, an isatin derivative with a methyl group as an electron-donating group at the 5-position gave (S)-13c in good yield, although with slightly decreased enantioselectivity. The introduction of the chloro group at the same position led to a moderate yield for (S)-13d, while the enantioselectivity remained high. In contrast, the reaction with the isatin derivative bearing a nitro group at the 5-position did not proceed, and (S)-13e was not produced. Likewise, no reaction occurred with a trifluoromethoxy-substituted derivative, resulting in no formation of (S)-13f. Reactions using isatin derivatives bearing halogen substituents at the 6-position proceeded efficiently, affording (S)-13g–i in good yields with high enantioselectivities. Conversely, the isatin derivative bearing a methoxy group at the 6-position led to a decreased yield for (S)-13j, though the enantioselectivity remained high. Additionally, we tested the reaction using an isatin derivative with a chloro group at the 7-position and obtained (S)-13k in good yield with moderate enantioselectivity. Furthermore, when (E)-1,3-di(p-chlorophenyl)-2-propenyl acetate (15) was utilized as an allylic acetate, the desired product (S)-13l was obtained in high yield with excellent enantioselectivity. We confirmed that the product 13 from the Pd-catalyzed asymmetric allylic amination of allyl esters with isatin using (aR)-(−)-6 possesses an S-configuration. This stereochemical outcome follows the same reaction mechanism as the Pd-catalyzed asymmetric allylic substitution of allyl esters with indoles using (aR)-(−)-6 [31]. To explore further applications of this product, we treated (S)-13a (94% ee) with malononitrile in the presence of FeCl3 as a catalyst [36] and obtained the corresponding malononitrile derivative (S)-16 without any loss of optical purity (Scheme 3).
Scheme 2: Pd-catalyzed asymmetric allylic amination of acetate 12 (Ar = Ph) or 15 (Ar = p-ClC6H4) with isatin derivatives 11 using (aR)-(−)-6 as a chiral ligand: The reaction was carried out at 0.1 mmol scale; yields refer to isolated yields.
Scheme 2: Pd-catalyzed asymmetric allylic amination of acetate 12 (Ar = Ph) or 15 (Ar = p-ClC6H4) with isatin...
Scheme 3: Transformation of the reaction product (S)-13a: The reaction was carried out at 0.1 mmol scale and the yield refers to the isolated yield.
Scheme 3: Transformation of the reaction product (S)-13a: The reaction was carried out at 0.1 mmol scale and ...
Conclusion
In this study, N-propyl-N-cinnamoylamide 7 was synthesized in three steps from phosphine oxide 8. Chiral HPLC analysis confirmed its axial chirality at the C(aryl)–N(amide) bond. The optical resolution of (±)-7 yielded (+)-7 and (−)-7. The racemization barrier of (−)-7 in n-dodecane was determined to be 25.0 kcal/mol at 25 °C, with a half-life of approximately 1.3 days. The chiral amides (aR)-(−)-6 and (−)-7 were evaluated as ligands in Pd-catalyzed asymmetric allylic amination, and while (−)-7 exhibited promising enantioselectivity, its yield was lower than (aR)-(−)-6. Further optimization of reaction conditions led to improved yields and enantioselectivities up to 95% ee. Moreover, the reaction was successfully scaled up to 1 mmol. The substrate scope was investigated using various isatin derivatives, yielding high enantioselectivities (up to 95% ee) for most, except for those bearing certain electron-withdrawing groups. Additionally, we demonstrated the further conversion of (S)-13a into the malononitrile derivative (S)-16 without loss of optical purity.
Supporting Information
Data of thermal racemization of 7, DFT calculations for investigating racemization mechanism of 7, general methods and materials, experimental procedures and characterization data, 1H, 13C and 31P NMR spectra for 9 and 10, 1H, 13C and 31P NMR spectra and HPLC charts for (±)-7, (+)-7 and (–)-7, 1H and 13C NMR spectra and HPLC charts for (S)-13a–k (except (S)-13e) and (S)-16.
| Supporting Information File 1: Experimental section and compounds characterization. | ||
| Format: PDF | Size: 13.0 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Ye, N.; Chen, H.; Wold, E. A.; Shi, P.-Y.; Zhou, J. ACS Infect. Dis. 2016, 2, 382–392. doi:10.1021/acsinfecdis.6b00041
Return to citation in text: [1] -
Santos, M. M. M. Tetrahedron 2014, 70, 9735–9757. doi:10.1016/j.tet.2014.08.005
Return to citation in text: [1] -
Williams, R. M.; Cox, R. J. Acc. Chem. Res. 2003, 36, 127–139. doi:10.1021/ar020229e
Return to citation in text: [1] -
Shmidt, M. S.; Reverdito, A. M.; Kremenchuzky, L.; Perillo, I. A.; Blanco, M. M. Molecules 2008, 13, 831–840. doi:10.3390/molecules13040831
Return to citation in text: [1] -
Coppola, G. M. J. Heterocycl. Chem. 1987, 24, 1249–1251. doi:10.1002/jhet.5570240503
Return to citation in text: [1] -
Imanzadeh, G.; Soltanizadeh, Z.; Khodayari, A.; Zamanloo, M.; Mansoori, Y.; Salehzadeh, J. Chin. J. Chem. 2012, 30, 891–899. doi:10.1002/cjoc.201100351
Return to citation in text: [1] -
Imanzadeh, G. H.; Mollaei Tavana, M.; Zamanloo, M. R.; Mansoori, Y. Chin. J. Chem. 2009, 27, 389–396. doi:10.1002/cjoc.200990064
Return to citation in text: [1] -
Cheng, Y.; Li, Z. J. Heterocycl. Chem. 2020, 57, 3574–3583. doi:10.1002/jhet.4075
Return to citation in text: [1] -
Žari, S.; Metsala, A.; Kudrjashova, M.; Kaabel, S.; Järving, I.; Kanger, T. Synthesis 2015, 47, 875–886. doi:10.1055/s-0034-1379956
Return to citation in text: [1] -
Žari, S.; Kudrjashova, M.; Pehk, T.; Lopp, M.; Kanger, T. Org. Lett. 2014, 16, 1740–1743. doi:10.1021/ol500421k
Return to citation in text: [1] -
Yang, W.; Du, D.-M. Chem. Commun. 2013, 49, 8842–8844. doi:10.1039/c3cc44930k
Return to citation in text: [1] -
Deepthi, K. L.; Subhashini, N. J. P.; Maneshwar, T. Asian J. Chem. 2022, 34, 1097–1104. doi:10.14233/ajchem.2022.23561
Return to citation in text: [1] -
Andreev, I. A.; Manvar, D.; Barreca, M. L.; Belov, D. S.; Basu, A.; Sweeney, N. L.; Ratmanova, N. K.; Lukyanenko, E. R.; Manfroni, G.; Cecchetti, V.; Frick, D. N.; Altieri, A.; Kaushik-Basu, N.; Kurkin, A. V. Eur. J. Med. Chem. 2015, 96, 250–258. doi:10.1016/j.ejmech.2015.04.022
Return to citation in text: [1] -
Zhang, X.; Zhang, J.-Q.; Sun, Z.-H.; Shan, H.-M.; Su, J.-C.; Ma, X.-P.; Su, G.-F.; Xu, L.-P.; Mo, D.-L. Angew. Chem., Int. Ed. 2025, 64, e202420390. doi:10.1002/anie.202420390
Return to citation in text: [1] -
Li, J.-J.; Zeng, X.-X.; Kuang, X.; Shen, H.-C.; Wang, P.; Yu, J.-Q. J. Am. Chem. Soc. 2025, 147, 6594–6603. doi:10.1021/jacs.4c15491
Return to citation in text: [1] -
Chen, Z.; Liu, J.; Ou, W.; Kato, T.; Wang, Z.; Chen, Y.; Liu, Y.; Maruoka, K. J. Org. Chem. 2024, 89, 12800–12811. doi:10.1021/acs.joc.4c01334
Return to citation in text: [1] -
Gavrilov, K. N.; Chuchelkin, I. V.; Trunina, V. M.; Gavrilov, B. K.; Firsin, I. D.; Rud, E. S.; Tafeenko, V. A.; Bermesheva, E. V. Russ. Chem. Bull. 2024, 73, 574–579. doi:10.1007/s11172-024-4167-0
Return to citation in text: [1] -
Gavrilov, K. N.; Chuchelkin, I. V.; Gavrilov, V. K.; Firsin, I. D.; Trunina, V. M.; Shiryaev, A. A.; Shkirdova, A. O.; Bermesheva, E. V.; Tafeenko, V. A.; Chernyshev, V. V.; Zimarev, V. S.; Goulioukina, N. S. Org. Biomol. Chem. 2024, 22, 6362–6369. doi:10.1039/d4ob00840e
Return to citation in text: [1] -
Gavrilov, K. N.; Chuchelkin, I. V.; Firsin, I. D.; Trunina, V. M.; Gavrilov, V. K.; Zheglov, S. V.; Fedorov, D. A.; Tafeenko, V. A.; Zamilatskov, I. A.; Zimarev, V. S.; Goulioukina, N. S. Org. Biomol. Chem. 2024, 22, 538–549. doi:10.1039/d3ob01891a
Return to citation in text: [1] -
Liu, M.; Zhang, X.; Bao, R.; Xiao, F.; Cen, S.; Zhang, Z. Org. Lett. 2023, 25, 5946–5950. doi:10.1021/acs.orglett.3c01972
Return to citation in text: [1] -
Gavrilov, K. N.; Chuchelkin, I. V.; Shiryaev, A. A.; Firsin, I. D.; Trunina, V. M.; Gavrilov, V. K.; Bityak, Y. P.; Fedorov, D. A.; Zimarev, V. S.; Goulioukina, N. S. Mendeleev Commun. 2023, 33, 776–778. doi:10.1016/j.mencom.2023.10.012
Return to citation in text: [1] -
Feng, B.; Fang, P.-W.; Lan, G.-M.; Peng, L.-Y.; Liang, L.-F.; You, G.-Y. Org. Biomol. Chem. 2022, 20, 7415–7418. doi:10.1039/d2ob01249a
Return to citation in text: [1] -
Gavrilov, K. N.; Chuchelkin, I. V.; Gavrilov, V. K.; Zheglov, S. V.; Firsin, I. D.; Trunina, V. M.; Zamilatskov, I. A.; Tyurin, V. S.; Tafeenko, V. A.; Chernyshev, V. V.; Zimarev, V. S.; Goulioukina, N. S. New J. Chem. 2022, 46, 1751–1762. doi:10.1039/d1nj05143a
Return to citation in text: [1] -
Gavrilov, K. N.; Chuchelkin, I. V.; Trunina, V. M.; Firsin, I. D.; Bityak, Y. P.; Fedorov, D. A.; Zimarev, V. S.; Goulioukina, N. S. Russ. J. Gen. Chem. 2022, 92, 2612–2619. doi:10.1134/s1070363222120088
Return to citation in text: [1] -
Firsin, I. D.; Chuchelkin, I. V.; Gavrilov, V. K.; Trunina, V. M.; Zimarev, V. S.; Zheglov, S. V.; Gavrilov, K. N.; Goulioukina, N. S. Phosphorus, Sulfur Silicon Relat. Elem. 2022, 197, 518–519. doi:10.1080/10426507.2021.1989691
Return to citation in text: [1] -
Lynch, C. C.; Balaraman, K.; Wolf, C. Org. Lett. 2020, 22, 3180–3184. doi:10.1021/acs.orglett.0c00936
Return to citation in text: [1] [2] -
Liu, Z.-S.; Hua, Y.; Gao, Q.; Ma, Y.; Tang, H.; Shang, Y.; Cheng, H.-G.; Zhou, Q. Nat. Catal. 2020, 3, 727–733. doi:10.1038/s41929-020-0494-1
Return to citation in text: [1] -
Mino, T.; Youda, J.; Ebisawa, T.; Shima, Y.; Nishikawa, K.; Yoshida, Y.; Sakamoto, M. J. Oleo Sci. 2018, 67, 1189–1199. doi:10.5650/jos.ess17260
Return to citation in text: [1] -
Mino, T.; Nishikawa, K.; Asano, M.; Shima, Y.; Ebisawa, T.; Yoshida, Y.; Sakamoto, M. Org. Biomol. Chem. 2016, 14, 7509–7519. doi:10.1039/c6ob01354f
Return to citation in text: [1] -
Mino, T.; Yamaguchi, D.; Masuda, C.; Youda, J.; Ebisawa, T.; Yoshida, Y.; Sakamoto, M. Org. Biomol. Chem. 2019, 17, 1455–1465. doi:10.1039/c9ob00075e
Return to citation in text: [1] -
Mino, T.; Takaya, K.; Koki, K.; Akimoto, N.; Yoshida, Y.; Kasashima, Y.; Sakamoto, M. Org. Biomol. Chem. 2023, 21, 2775–2778. doi:10.1039/d3ob00224a
Return to citation in text: [1] [2] [3] -
Mino, T.; Tanaka, Y.; Sakamoto, M.; Fujita, T. Tetrahedron: Asymmetry 2001, 12, 2435–2440. doi:10.1016/s0957-4166(01)00426-8
Return to citation in text: [1] -
Cooke, A. S.; Harris, M. M. J. Chem. Soc. C 1967, 988–992. doi:10.1039/j39670000988
Return to citation in text: [1] -
Cagle, F. W., Jr.; Eyring, H. J. Am. Chem. Soc. 1951, 73, 5628–5630. doi:10.1021/ja01156a038
Return to citation in text: [1] -
Eyring, H. Chem. Rev. 1935, 17, 65–77. doi:10.1021/cr60056a006
Return to citation in text: [1] -
Huang, L.-S.; Lai, Y.-H.; Yang, C.; Xu, D.-Z. Appl. Organomet. Chem. 2019, 33, e4910. doi:10.1002/aoc.4910
Return to citation in text: [1]
| 36. | Huang, L.-S.; Lai, Y.-H.; Yang, C.; Xu, D.-Z. Appl. Organomet. Chem. 2019, 33, e4910. doi:10.1002/aoc.4910 |
| 26. | Lynch, C. C.; Balaraman, K.; Wolf, C. Org. Lett. 2020, 22, 3180–3184. doi:10.1021/acs.orglett.0c00936 |
| 31. | Mino, T.; Takaya, K.; Koki, K.; Akimoto, N.; Yoshida, Y.; Kasashima, Y.; Sakamoto, M. Org. Biomol. Chem. 2023, 21, 2775–2778. doi:10.1039/d3ob00224a |
| 1. | Ye, N.; Chen, H.; Wold, E. A.; Shi, P.-Y.; Zhou, J. ACS Infect. Dis. 2016, 2, 382–392. doi:10.1021/acsinfecdis.6b00041 |
| 2. | Santos, M. M. M. Tetrahedron 2014, 70, 9735–9757. doi:10.1016/j.tet.2014.08.005 |
| 3. | Williams, R. M.; Cox, R. J. Acc. Chem. Res. 2003, 36, 127–139. doi:10.1021/ar020229e |
| 9. | Žari, S.; Metsala, A.; Kudrjashova, M.; Kaabel, S.; Järving, I.; Kanger, T. Synthesis 2015, 47, 875–886. doi:10.1055/s-0034-1379956 |
| 10. | Žari, S.; Kudrjashova, M.; Pehk, T.; Lopp, M.; Kanger, T. Org. Lett. 2014, 16, 1740–1743. doi:10.1021/ol500421k |
| 11. | Yang, W.; Du, D.-M. Chem. Commun. 2013, 49, 8842–8844. doi:10.1039/c3cc44930k |
| 33. | Cooke, A. S.; Harris, M. M. J. Chem. Soc. C 1967, 988–992. doi:10.1039/j39670000988 |
| 34. | Cagle, F. W., Jr.; Eyring, H. J. Am. Chem. Soc. 1951, 73, 5628–5630. doi:10.1021/ja01156a038 |
| 35. | Eyring, H. Chem. Rev. 1935, 17, 65–77. doi:10.1021/cr60056a006 |
| 6. | Imanzadeh, G.; Soltanizadeh, Z.; Khodayari, A.; Zamanloo, M.; Mansoori, Y.; Salehzadeh, J. Chin. J. Chem. 2012, 30, 891–899. doi:10.1002/cjoc.201100351 |
| 7. | Imanzadeh, G. H.; Mollaei Tavana, M.; Zamanloo, M. R.; Mansoori, Y. Chin. J. Chem. 2009, 27, 389–396. doi:10.1002/cjoc.200990064 |
| 8. | Cheng, Y.; Li, Z. J. Heterocycl. Chem. 2020, 57, 3574–3583. doi:10.1002/jhet.4075 |
| 31. | Mino, T.; Takaya, K.; Koki, K.; Akimoto, N.; Yoshida, Y.; Kasashima, Y.; Sakamoto, M. Org. Biomol. Chem. 2023, 21, 2775–2778. doi:10.1039/d3ob00224a |
| 5. | Coppola, G. M. J. Heterocycl. Chem. 1987, 24, 1249–1251. doi:10.1002/jhet.5570240503 |
| 31. | Mino, T.; Takaya, K.; Koki, K.; Akimoto, N.; Yoshida, Y.; Kasashima, Y.; Sakamoto, M. Org. Biomol. Chem. 2023, 21, 2775–2778. doi:10.1039/d3ob00224a |
| 4. | Shmidt, M. S.; Reverdito, A. M.; Kremenchuzky, L.; Perillo, I. A.; Blanco, M. M. Molecules 2008, 13, 831–840. doi:10.3390/molecules13040831 |
| 32. | Mino, T.; Tanaka, Y.; Sakamoto, M.; Fujita, T. Tetrahedron: Asymmetry 2001, 12, 2435–2440. doi:10.1016/s0957-4166(01)00426-8 |
| 26. | Lynch, C. C.; Balaraman, K.; Wolf, C. Org. Lett. 2020, 22, 3180–3184. doi:10.1021/acs.orglett.0c00936 |
| 28. | Mino, T.; Youda, J.; Ebisawa, T.; Shima, Y.; Nishikawa, K.; Yoshida, Y.; Sakamoto, M. J. Oleo Sci. 2018, 67, 1189–1199. doi:10.5650/jos.ess17260 |
| 29. | Mino, T.; Nishikawa, K.; Asano, M.; Shima, Y.; Ebisawa, T.; Yoshida, Y.; Sakamoto, M. Org. Biomol. Chem. 2016, 14, 7509–7519. doi:10.1039/c6ob01354f |
| 14. | Zhang, X.; Zhang, J.-Q.; Sun, Z.-H.; Shan, H.-M.; Su, J.-C.; Ma, X.-P.; Su, G.-F.; Xu, L.-P.; Mo, D.-L. Angew. Chem., Int. Ed. 2025, 64, e202420390. doi:10.1002/anie.202420390 |
| 15. | Li, J.-J.; Zeng, X.-X.; Kuang, X.; Shen, H.-C.; Wang, P.; Yu, J.-Q. J. Am. Chem. Soc. 2025, 147, 6594–6603. doi:10.1021/jacs.4c15491 |
| 16. | Chen, Z.; Liu, J.; Ou, W.; Kato, T.; Wang, Z.; Chen, Y.; Liu, Y.; Maruoka, K. J. Org. Chem. 2024, 89, 12800–12811. doi:10.1021/acs.joc.4c01334 |
| 17. | Gavrilov, K. N.; Chuchelkin, I. V.; Trunina, V. M.; Gavrilov, B. K.; Firsin, I. D.; Rud, E. S.; Tafeenko, V. A.; Bermesheva, E. V. Russ. Chem. Bull. 2024, 73, 574–579. doi:10.1007/s11172-024-4167-0 |
| 18. | Gavrilov, K. N.; Chuchelkin, I. V.; Gavrilov, V. K.; Firsin, I. D.; Trunina, V. M.; Shiryaev, A. A.; Shkirdova, A. O.; Bermesheva, E. V.; Tafeenko, V. A.; Chernyshev, V. V.; Zimarev, V. S.; Goulioukina, N. S. Org. Biomol. Chem. 2024, 22, 6362–6369. doi:10.1039/d4ob00840e |
| 19. | Gavrilov, K. N.; Chuchelkin, I. V.; Firsin, I. D.; Trunina, V. M.; Gavrilov, V. K.; Zheglov, S. V.; Fedorov, D. A.; Tafeenko, V. A.; Zamilatskov, I. A.; Zimarev, V. S.; Goulioukina, N. S. Org. Biomol. Chem. 2024, 22, 538–549. doi:10.1039/d3ob01891a |
| 20. | Liu, M.; Zhang, X.; Bao, R.; Xiao, F.; Cen, S.; Zhang, Z. Org. Lett. 2023, 25, 5946–5950. doi:10.1021/acs.orglett.3c01972 |
| 21. | Gavrilov, K. N.; Chuchelkin, I. V.; Shiryaev, A. A.; Firsin, I. D.; Trunina, V. M.; Gavrilov, V. K.; Bityak, Y. P.; Fedorov, D. A.; Zimarev, V. S.; Goulioukina, N. S. Mendeleev Commun. 2023, 33, 776–778. doi:10.1016/j.mencom.2023.10.012 |
| 22. | Feng, B.; Fang, P.-W.; Lan, G.-M.; Peng, L.-Y.; Liang, L.-F.; You, G.-Y. Org. Biomol. Chem. 2022, 20, 7415–7418. doi:10.1039/d2ob01249a |
| 23. | Gavrilov, K. N.; Chuchelkin, I. V.; Gavrilov, V. K.; Zheglov, S. V.; Firsin, I. D.; Trunina, V. M.; Zamilatskov, I. A.; Tyurin, V. S.; Tafeenko, V. A.; Chernyshev, V. V.; Zimarev, V. S.; Goulioukina, N. S. New J. Chem. 2022, 46, 1751–1762. doi:10.1039/d1nj05143a |
| 24. | Gavrilov, K. N.; Chuchelkin, I. V.; Trunina, V. M.; Firsin, I. D.; Bityak, Y. P.; Fedorov, D. A.; Zimarev, V. S.; Goulioukina, N. S. Russ. J. Gen. Chem. 2022, 92, 2612–2619. doi:10.1134/s1070363222120088 |
| 25. | Firsin, I. D.; Chuchelkin, I. V.; Gavrilov, V. K.; Trunina, V. M.; Zimarev, V. S.; Zheglov, S. V.; Gavrilov, K. N.; Goulioukina, N. S. Phosphorus, Sulfur Silicon Relat. Elem. 2022, 197, 518–519. doi:10.1080/10426507.2021.1989691 |
| 30. | Mino, T.; Yamaguchi, D.; Masuda, C.; Youda, J.; Ebisawa, T.; Yoshida, Y.; Sakamoto, M. Org. Biomol. Chem. 2019, 17, 1455–1465. doi:10.1039/c9ob00075e |
| 13. | Andreev, I. A.; Manvar, D.; Barreca, M. L.; Belov, D. S.; Basu, A.; Sweeney, N. L.; Ratmanova, N. K.; Lukyanenko, E. R.; Manfroni, G.; Cecchetti, V.; Frick, D. N.; Altieri, A.; Kaushik-Basu, N.; Kurkin, A. V. Eur. J. Med. Chem. 2015, 96, 250–258. doi:10.1016/j.ejmech.2015.04.022 |
| 12. | Deepthi, K. L.; Subhashini, N. J. P.; Maneshwar, T. Asian J. Chem. 2022, 34, 1097–1104. doi:10.14233/ajchem.2022.23561 |
| 27. | Liu, Z.-S.; Hua, Y.; Gao, Q.; Ma, Y.; Tang, H.; Shang, Y.; Cheng, H.-G.; Zhou, Q. Nat. Catal. 2020, 3, 727–733. doi:10.1038/s41929-020-0494-1 |
© 2025 Akimoto et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.