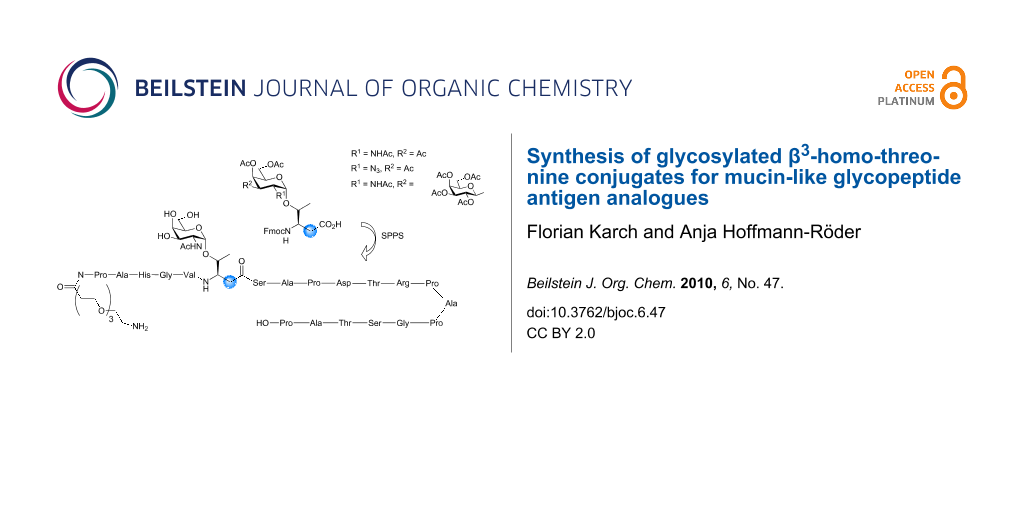
Abstract
Glycopeptides from the mucin family decorated with tumour-associated carbohydrate antigens (TACA) have proven to be important target structures for the development of molecularly defined anti-cancer vaccines. The strategic incorporation of β-amino acid building blocks into such mucin-type sequences offers the potential to create pseudo-glycopeptide antigens with improved bioavailability for tumour immunotherapy. Towards this end, TN and TF antigen conjugates O-glycosidically linked to Fmoc-β3-homo-threonine were prepared in good yield via Arndt–Eistert homologation of the corresponding glycosyl α-amino acid derivative. By incorporation of TN-Fmoc-β3hThr conjugate into the 20 amino acid tandem repeat sequence of MUC1 using sequential solid-phase glycopeptide synthesis, a first example of a mixed α/β-hybrid glycopeptide building block was obtained. The latter is of interest for the development of novel glycoconjugate mimics and model structures for anti-cancer vaccines with increased biological half-life.
Graphical Abstract
Introduction
Glycosylation is the predominant co- and post-translational modification in higher organisms responsible for tailoring and fine-tuning of the activity of proteins involved in fundamental biological recognition events of cell adhesion, cell differentiation and cell growth [1-3]. As a consequence, synthetic oligosaccharides and their conjugates are recognised as important tools for the expanding field of chemical biology [4]. Aberrant glycosylation of cell surface glycoproteins is associated with various pathological incidents, e.g., autoimmune and infectious diseases and cancer. In the latter case, unusual glycan structures composed of truncated O-linked oligosaccharides of carcinoma-derived mucin glycoproteins can be used as markers of the tumourigenic process and as target structures for cancer immunotherapy [5]. Over the last years, mucin-type glycopeptides decorated with tumour-associated carbohydrate antigens (TACA) have been shown to trigger strong humoral immunity within molecularly defined vaccine prototypes [6-10]. However, the limited metabolic stability of the glycopeptide conjugates represents a major obstacle for the development of efficient carbohydrate-based vaccines. Various strategies towards the incorporation of non-natural hydrolysis resistant carbohydrate analogues into vaccine constructs have been pursued. For instance, stable TACA mimics comprising C-glycosides [11-14], S-glycosides [15-19] and deoxyfluoro sugars [20] have been used to circumvent hydrolytic degradation by endogenous glycosidases.
In principle, antigenicity of the artificial TACA derivatives should be enhanced by minor structural modifications assuming that the conformations remain similar to those of the natural antigens. In this respect, hybrid peptides in which β3-homo-amino acids are used to strategically replace α-amino acids might be of interest as platforms for carbohydrate-based vaccines. That is because such mixed α/β-peptides adopt stable secondary structures closely related to those of natural α-peptides [21,22]. Moreover, inclusion of a single β-amino acid into an α-peptidic sequence already augments local and/or general stability against proteolytic degradation in vitro and in vivo; thus enabling the development of diverse peptidomimetics for an increasing number of applications [22-25]. Therefore we contemplated the use of mucin-derived α/β-hybrid glycopeptides as stable mimetics of naturally occurring glycocopeptide antigens for cancer vaccines. We were surprised to see how little precedence was available for this approach. Besides a recent report on α/δ-hybrid peptides derived from Neu2en and L-Glu [26], representing potentially immunogenic mimics of α-2,8-linked polysialic acid, only a few β-glycopeptides comprising N-acetylglucosamine [27-29] and N-acetylgalactosamine [30,31] (TN antigen) linked to β3-homo-serine are known. Despite their importance as specific tumour antigens, conjugates of Fmoc-β3hSer and Fmoc-β3hThr carrying larger TACA structures such as the Thomsen–Friedenreich antigen (TF) or its sialylated variants (α2-6sTF and α2-3sTF) have not been reported to date.
By presenting orthogonally protected TN and TF antigen conjugates of Fmoc-β3hThr (Figure 1) as well as a first α/β-hybrid glycopeptide analogue comprising the 20 amino acid tandem repeat sequence of the human mucin MUC1, we describe preliminary results of our synthetic efforts towards the preparation of mucin-type glycopeptide mimetics.
Figure 1: Structures of the naturally occurring TN and TF antigens and the targeted Fmoc-β3hThr analogues.
Figure 1: Structures of the naturally occurring TN and TF antigens and the targeted Fmoc-β3hThr analogues.
Results and Discussion
Initial attempts to directly link the carbohydrate entity to the β-side chain of a preformed Fmoc-β3hThr conjugate were unsuccessful due to rapid lactonisation [29]. Similarly, despite the use of various glycosyl donors and reaction conditions, glycosylation of the corresponding dipeptide precursor Fmoc-β3hThr(OH)-Ala-OBn failed completely. Therefore we encountered the strategy of Arndt–Eistert homologation in the synthesis of the target Fmoc-β3hThr(αAc3GalNAc) and Fmoc-β3hThr(β(Ac4Gal(1–3))α(Ac3GalNAc)) conjugates 2a and 4, respectively, as reported by Norgren et al. [29]. TN antigen derivative Fmoc-Thr(αAc3GalNAc)-OH (1a) was prepared according to published procedures [32,33] and converted into the corresponding diazo ketone upon treatment with isobutyl chloroformate in the presence of N-methylmorpholine (NMM) and diazomethane (Scheme 1). Without further purification, the latter was subjected to a silver-promoted Wolff-rearrangement, again using NMM as the base, providing the spectroscopically pure TN antigen analogue Fmoc-β3hThr(αAc3GalNAc)-OH (2a) in 60% yield over two steps after aqueous work-up.
Scheme 1: Synthesis of Fmoc-β3hThr antigen conjugates by Arndt–Eistert homologation.
Scheme 1: Synthesis of Fmoc-β3hThr antigen conjugates by Arndt–Eistert homologation.
Compound 2a was also accessible from a direct synthetic precursor of TN derivative 1a in which the 2-acetamido substituent was masked by an azido group. Thus, upon subjection of Fmoc-Thr(αAc3GalN3)-OH (1b) [32] to the same homologation sequence as before, the corresponding β3hThr analogue 2b was obtained in 82% yield. Subsequent zinc-mediated reduction and acetylation led to the formation of conjugate 2a in 56% yield.
During biosynthesis TN antigen acts as an immediate precursor of the TF antigen. As a consequence, a biomimetic approach towards larger TACA structures via stepwise assembly of the glycan chain has been pursued in various antigen syntheses [33,34]. By applying chemical or enzymatic 3β-galactosylation, the 3-OH deprotected conjugate 2b could be converted into the desired antigen derivative Fmoc-β3hThr(β(Ac4Gal(1–3))α(Ac3GalNAc))-OH (4). While this strategy certainly requires the use of optimised protecting group manipulations and glycosylation protocols, the alternative route to compound 4 via Arndt–Eistert homologation would benefit from an established and reliable synthesis of key building block 3 [32,33]. To our delight, the homologation reaction of glycosyl amino acid 3 again proceeded smoothly to afford the desired TF-β3hThr conjugate 4 in spectroscopically pure form and good chemical yield after aqueous work-up (Scheme 1).
To demonstrate the usefulness of the novel glycosylated β3hThr conjugates as antigen mimics, TN antigen analog 2a was incorporated into an α/β-hybrid glycopeptide 7 comprising a full tandem repeat sequence of the epithelial mucin MUC1 and an N-terminal non-immunogenic triethylene glycol spacer. The latter can be used for further conjugation to immunostimulants (e.g., BSA [35] or tetanus toxoid [36]) and for immobilisation onto microarray platforms [37] within functional immunological studies. The MUC1 pseudo-glycopeptide was assembled in an automated synthesiser by the Fmoc-strategy on a TentaGel S resin 5 equipped with a bulky trityl linker [38] to avoid diketopiperazine formation and pre-loaded with Fmoc-proline (Scheme 2). The first 13 amino acids of the MUC1 sequence were coupled under standard conditions using piperidine in N-methylpyrrolidone (NMP) to remove the temporary Fmoc protecting group followed by coupling of excess (10 equiv) Fmoc-amino acid activated by HBTU/HOBt [39] and diisopropylethylamine (DIPEA) in DMF. Unreacted amino acids were capped after each cycle with Ac2O in the presence of DIPEA and catalytic amounts of HOBt in NMP. The sterically demanding glycosylated β3hthreonine building block 2a (1.5 equiv), was coupled over an extended reaction time of 8 h employing the more reactive reagents HATU/HOAt [40] with N-methylmorpholine (NMM) in NMP for activation. After the final five Fmoc-amino acids of the TR-sequence were coupled according to the standard protocol, a triethylene glycol spacer 6 [41] (10 equiv) was attached using the standard coupling procedure, again. Simultaneous detachment of the glycopeptide from the resin and cleavage of the acid-labile amino acid side chain protective groups was achieved upon treatment with a mixture of TFA, triisopropylsilane and water. The resulting partially deblocked glycopeptide 7 was isolated after purification by semi-preparative RP-HPLC in a yield of 36%, based on the loaded resin 5. The final de-O-acetylation of the glycan portion was accomplished upon prolonged treatment with catalytic amounts of NaOMe in methanol at pH 9.5 to afford glycopeptide 8 in 18% yield (based on the loaded resin) after semi-preparative RP-HPLC.
Scheme 2: Solid-phase synthesis of the tumour-associated MUC1 α/β-hybrid glycopeptide analogue 8 carrying the TN antigen glycan. NMP = N-methylpyrrolidone, HBTU = O-(1H-benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate, HOBt = N-hydroxybenzotriazole, DIPEA = diisopropylethylamine, HATU = O-(7-azabenzotriazole-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate, HOAt = N-hydroxy-7-azabenzotriazole, NMM = N-methylmorpholine, TIS = triisopropylsilane.
Scheme 2: Solid-phase synthesis of the tumour-associated MUC1 α/β-hybrid glycopeptide analogue 8 carrying the...
Conclusion
Two novel tumour-associated carbohydrate antigen analogues with TN and TF determinants O-glycosidically linked to the side chain of Fmoc-β3hThr-OH have been prepared by Arndt–Eistert homologation of the corresponding glycosylated α-amino acids 1a and 1b. The resulting β3hThr glycoconjugates are valuable antigen mimetics with potentially enhanced chemical and metabolic stability. They might serve as precursors for the preparation of further mucin-type antigen structures, e.g., sialylated ones or those based on core2 structures. In addition, the preparation of a first MUC1 pseudo-glycopeptide comprising the modified glycosyl amino acid Fmoc-β3hThr(αGalNAc)-OH at position Thr15 has been accomplished using solid-phase peptide synthesis. By appropriate conjugation, the resulting α/β-hybrid glycopeptide conjugate could be used as an antigen surrogate to elucidate the effects of chemically modified antibody determinants on the immunological properties of glycopeptide antigen analogues.
Experimental
General remarks: DMF (amine-free, for peptide synthesis) and NMP were purchased from Roth and Ac2O in p.a. quality from Acros. Fmoc-protected amino acids were purchased from Orpegen Pharma. For solid-phase synthesis, pre-loaded TentaGel S resin (Rapp Polymere) was employed. Reactions were monitored by TLC with pre-coated silica gel 60 F254 aluminium plates (Merck KGaA, Darmstadt). HPLC analyses were performed on a JASCO-HPLC system with Phenomenex Luna C18(2) (250 × 4.6 mm, 10 μm) and Phenomenex Jupiter C18(2) (250 × 4.6 mm, 10 μm) columns at a flow rate of 1 mL min−1. Preparative RP-HPLC separation was carried out on a JASCO-HPLC System with Phenomenex Luna C18(2) (250 × 30 mm, 10 μm) and Phenomenex Jupiter C18(2) (250 × 30 mm, 10 μm) columns at a flow rate of 20 mL min−1 or 10 mL min−1. 1H, 13C and 2D NMR spectra were recorded on a Bruker AC-300 or a Bruker AM-400 spectrometer. The chemical shifts are reported in ppm relative to the signal of the deuterated solvent. Multiplicities are given as: s (singlet), br s (broad singlet), d (doublet), t (triplet) and m (multiplet). In the case of known compounds, all spectra obtained were consistent with the literature. HR-ESI-mass spectra were recorded on a Micromass Q TOF Ultima 3 spectrometer and optical rotations were measured at 546 nm with a Perkin-Elmer polarimeter 241.
General procedure for the synthesis of diazomethane in ethereal solution
Caution: Diazomethane is toxic, highly-volatile, cancerogenic and explosive. Its generation and handling thus requires special precautions [42]. With regard to the free acids, 10 equiv of N-methyl-N-nitroso-urea were added to a solution of 50% KOH in H2O which was layered on top with Et2O. The organic layer was decanted and replaced by a new portion of Et2O until the organic layer was no longer coloured. The united organic phases were dried over KOH at −25 °C for 3 h.
General procedure (GP1) for the synthesis of diazo ketones: The free acid (1 equiv) was dissolved in 2 mL of dry THF under an argon atmosphere. At −25 °C, 1 equiv of NMM and 1 equiv of isobutyl chloro formate were added subsequently and the resulting suspension was stirred for 20 min at this temperature. The mixture was allowed to reach 0 °C and the diazomethane solution in Et2O was added. The yellow solution was stirred 20 min at 0 °C before it was allowed to reach room temperature and stirred for further 16 h. Excess of diazomethane was destroyed by adding a few drops of acetic acid to the solution until no further nitrogen formation was observed. The solvents were removed under reduced pressure and the residue was dissolved in 20 mL Et2O. The organic layer was washed twice with saturated aqueous NaHCO3, saturated aqueous NH4Cl and brine. The organic layer was dried over Na2SO4, filtered, and the solvents were removed under reduced pressure. The resulting diazo ketones were used without further purification.
General procedure (GP2) for the Wolff-rearrangement: The diazo ketone (1 equiv) was dissolved in a mixture of THF/H2O (9:1) and was cooled to 0 °C. A solution of 0.11 equiv silver trifluoroacetate in 2.3 equiv NMM was added, and the mixture was stirred for 16 h while it was allowed to warm to room temperature. After evaporation of THF, the aqueous layer was diluted with saturated aqueous NaHCO3 and Et2O was added. The organic layer was extracted three times with saturated aqueous NaHCO3. The aqueous phases were collected, cooled to 0 °C and acidified to pH 1. The resulting suspension was extracted five times with Et2O. The organic layer was dried over Na2SO4 and evaporated in vacuo.
N-(9H-fluoren-9-yl)methoxycarbonyl-[α-3,4,6-tri-O-acetyl-2-acetamido-2-deoxy-galactopyranosyl]-β3-homo-threonine 2a: Procedure A: The synthesis followed the general procedures GP1 and GP2. Amounts: 150 mg (0.22 mmol) 1a. Yield: 90 mg (0.13 mmol), 60%, colourless amorphous solid. Analytical RP-HPLC (Luna, MeCN–H2O + 0.1% TFA, 20:80 → 60:40, 40 min, tR = 29.8 min). Procedure B: To a stirred solution of 2b (860 mg, 0.48 mmol) in a mixture of THF–Ac2O–AcOH (3:2:1, 36 mL) activated zinc dust (0.6 g, 9.1 mmol) was added. Activation was achieved by suspension in aq. 2% solution of CuSO4, followed by subsequent washings with water, EtOAc and Et2O. The reaction mixture was stirred for 16 h at room temperature, diluted with 50 mL THF and filtered through Hyflo Supercel®. The filtrate was concentrated in vacuo and co-evaporated five times with toluene and CH2Cl2. The residue was dissolved in 50 mL CH2Cl2, washed with saturated aqueous NaHCO3 and brine, dried (Na2SO4) and concentrated in vacuo. Yield: 328 mg (0.48 mmol), 56%, colourless amorphous solid; [α]D23 = 42.6 (c = 1.00, CHCl3); 1H NMR (COSY, HSQC, 400 MHz, CD3OD), δ (ppm) = 7.81 (d, 2 H, J = 7.2 Hz, Fmoc-H4, Fmoc-H5), 7.66, 7.65 (2d, 2 H, J = 7.6 Hz, Fmoc-H1, Fmoc-H8), 7.39 (pt, 2 H, Fmoc-H3, Fmoc-H6), 7.32 (t, 2 H, J = 7.6 Hz, Fmoc-H2, Fmoc-H7), 5.44 (d, 1 H, J = 2.4 Hz, GalNAc-H4), 5.10 (dd, 1 H, J = 3.2 Hz, 11.6 Hz, GalNAc-H3), 4.98 (d, 1 H, J = 3.6 Hz, GalNAc-H1), 4.56 (pdd, 1 H, Fmoc-CH2), 4.45 (dd, 1 H, J = 3.6 Hz, 11.6 Hz, GalNAc-H2), 4.41–4.35 (m, 1 H, Fmoc-CH2), 4.31 (pt, 1 H, GalNAc-H5), 4.21 (pt, 1 H, Fmoc-H9), 4.15–4.07 (m, 2 H, GalNAc-H6), 4.05–4.02 (m, 1 H, hTβ), 3.83 (pdd, 1 H, hTγ), 2.55 (pdd, 1 H, hTα), 2.38 (pdd, 1 H, hTα), 1.76 (d, 3 H, J = 6.4 Hz, hTδ); 13C NMR (DEPT, HSQC, 100.6 MHz, CD3OD), δ (ppm) = 175.1, 172.2, 170.7, 170.7, 170.6 (C=O), 157.0 (C=O-urethane), 144.0, 143.8 (Fmoc-C1a, Fmoc-C8a), 141.3, 141.3 (Fmoc-C4a, Fmoc-C5a), 127.4 (Fmoc-C3, Fmoc-C6), 126.8, 126.7 (Fmoc-C2, Fmoc-C7), 124.7, 124.6 (Fmoc-C1, Fmoc-C8), 119.6, 119.5 (Fmoc-C4, Fmoc-C5), 99.1 (GalNAc-C1), 77.6 (hTγ), 68.3 (GalNAc-C3), 67.4 (GalNAc-C4), 66.6 (GalNAc-C5), 65.9 (Fmoc-CH2), 61.8 (GalNAc-C6), 52.6 (hTβ), 47.6 (GalNAc-C2), 47.3 (Fmoc-C9), 36.8 (hTα), 21.3 (CH3-NHAc), 19.3, 19.2, 19.1 (CH3-Ac), 16.7 (hTδ); HR-ESI-MS (positive, m/z): 707.2433 ([M+Na]+, calc.: 707.2428).
N-(9H-fluoren-9-yl)methoxycarbonyl-[α-3,4,6-tri-O-acetyl-2-azido-2-deoxy-galactopyranosyl]-β3-homo-threonine 2b: The synthesis followed the general procedures GP1 and GP2. Amounts: 156 mg (0.23 mmol) 1b. Yield: 130 mg (0.19 mmol), 82%, colourless amorphous solid; [α]D23 = 56.0 (c = 1.00, CHCl3); 1H NMR (400 MHz, COSY, HSQC, CD3OD), δ (ppm) = 7.79 (d, 2 H, J = 7.6 Hz, Fmoc-H4, Fmoc-H5), 7.66 (d, 2 H, J = 7.6 Hz, Fmoc-H1, Fmoc-H8), 7.39 (t, 2 H, J = 7.2 Hz, Fmoc-H3, Fmoc-H6), 7.32 (t, 2 H, J = 7.2 Hz, Fmoc-H2, Fmoc-H7), 5.44 (d, 1 H, J = 2.0 Hz, GalNAc-H4), 5.33 (dd, 1 H, J = 3.2 Hz, 11.2 Hz, GalNAc-H3), 5.14 (d, 1 H, J = 3.6 Hz, GalNAc-H1), 4.41–4.32 (m, 3 H, Fmoc-CH2, Fmoc-H9), 4.21 (pt, 1 H, GalNAc-H5), 4.14–4.06 (m, 3 H, GalNAc-H6, hTβ), 3.91–3.86 (m, 2 H, GalNAc-H2, hTγ), 2.76 (dd, 1 H, J = 5.2 Hz, 16 Hz, hTα), 2.55 (dd, 1 H, J = 8.8 Hz, 16.4 Hz, hTα), 2.14, 2.02, 1.98 (3s, 9 H, 3 × CH3-Ac); 1.76 (d, 3 H, J = 6.4 Hz, hTδ); 13C NMR (101 MHz, DEPT, HSQC, CD3OD), δ (ppm) = 173.5, 170.7, 170.6, 170.1 (C=O), 157.1 (C=O-urethane), 144.0, 143.9 (Fmoc-C1a, Fmoc-C8a), 141.2 (Fmoc-C4a, Fmoc-C5a), 127.4 (Fmoc-C3, Fmoc-C6), 126.8, 126.7 (Fmoc-C2, Fmoc-C7), 124.9 (Fmoc-C1, Fmoc-C8), 119.5 (Fmoc-C4, Fmoc-C5), 98.6 (GalNAc-C1), 77.5 (hTγ), 69.0 (GalNAc-C3), 67.7 (GalNAc-C4), 66.8 (GalNAc-C5), 66.4 (Fmoc-CH2), 58.4 (GalNAc-C6), 52.0 (hTγ), 47.6 (GalNAc-C2), 47.3 (Fmoc-C9), 34.8 (hTα), 19.3, 19.2, 19.1 (CH3-Ac), 16.4 (hTδ); HR-ESI-MS (positive, m/z): 691.2232 ([M+Na]+ calc.: 691.2227).
N-(9H-fluoren-9-yl)methoxycarbonyl-[(β-2,3,4,6-tetra-O-acetylgalactopyranosyl)-(1–3)-(α-2-acetamido-4,6-di-O-acetyl-2-deoxy-galactopyranosyl)]-β3-homo-threonine 4: The synthesis followed the general procedures GP1 and GP2. Amounts: 200 mg (0.21 mmol) 3. Yield: 130.3 mg (0.13 mmol), 64%, colourless amorphous solid; [α]D23 = 29.9 (c = 0.50, CHCl3); 1H NMR (400 MHz, COSY, HSQC, CD3OD), δ (ppm) = 7.85 (d, 2 H, J = 7.6 Hz, Fmoc-H4, Fmoc-H5), 7.69 (d, 2 H, J = 7.6 Hz, Fmoc-H1, Fmoc-H8), 7.44 (t, 2 H, J = 7.6 Hz, Fmoc-H3, Fmoc-H6), 7.36 (t, 2 H, J = 7.6 Hz, Fmoc-H2, Fmoc-H7), 5.43 (d, 1 H, J = 3.4 Hz, GalNAc-H4), 5.39 (m, 1 H, Gal-H3), 5.04–5.02 (m, 2 H, Gal-H4, Gal-H2), 4.98 (under H2O-peak, GalNAc-H1), 4.71 (m, 1H, Gal-H1), 4.51 (d, 2 H, J = 6.2 Hz, Gal-H6), 4.44 (dd, 1 H, J = 3.5 Hz, 11.5 Hz, GalNAc-H2), 4.28–4.23 (m, 2 H, Gal-H5, Fmoc-H9), 4.23–4.21 (m, 3 H, Fmoc-CH2, GalNAc-H6), 4.09–3.91 (m, 4 H, GalNAc-H5, Fmoc-CH2, hTβ, GalNAc-H3), 3.86 (dd, 1 H, J = 2.7 Hz, 6.2 Hz, hTγ), 2.71 (dd, 1 H, J = 6.3 Hz, 15.5 Hz, hTα), 2.55 (dd, 1 H, J = 8.7 Hz, 15.6 Hz, hTα), 2.17, 2.15, 2.06, 2.06, 2.05, 2.04, 1.97 (7s, 21 H, 6 × CH3-Ac, CH3-AcNH), 1.22 (d, 3 H, J = 6.6 Hz, hTδ); 13C NMR (101 MHz, DEPT, HSQC, CD3OD), δ (ppm) = 174.7, 173.0, 172.5, 172.2, 171.6, 171.2 (C=O), 158.5 (C=O-urethane), 145.5, 145.3 (Fmoc-C1a, Fmoc-C8a), 142.8, 142.8 (Fmoc-C4a, Fmoc-C5a), 129.0, 128.4 (Fmoc-C3, Fmoc-C6), 126.3, 126.2 (Fmoc-C2, Fmoc-C7), 121.2 (Fmoc-C1, Fmoc-C8, Fmoc-C4, Fmoc-C5), 102.6 (Gal-C1), 100.6 (GalNAc-C1), 78.2 (hTγ), 74.9 (GalNAc-C3), 72.2 (Gal-C4), 71.8 (GalNAc-C5), 71.2 (GalNAc-C4), 70.1 (Gal-C2), 68.8 (Gal-C5), 68.5 (Gal-C3), 67.4 (Gal-C6), 64.3 (Fmoc-CH2), 62.3 (GalNAc-C6), 53.6 (hTβ), 50.3 (GalNAc-C2), 36.5 (hTα), 23.0 (CH3-NHAc), 20.8, 20.8, 20.7, 20.5 (CH3-Ac), 18.0 (hTδ); HR-ESI-MS (positive, m/z): 1159.3216 ([M+Na]+, calc.: 1159.3210).
Amino-4,7,10-trioxadodecanylamido-N-L-prolyl-L-alanyl-L-histidyl-L-glycyl-L-valyl-O-[α-3,4,6-tri-O-acetyl-2-acetamido-2-deoxy-galactopyranosyl]-homo-β3-threonyl-L-seryl-L-alanyl-L-prolyl-L-aspartyl-L-threonyl-L-arginyl-L-prolyl-L-alanyl-L-prolyl-L-glycyl-L-seryl-L-threonyl-L-alanyl-L-proline 7: The synthesis was carried out in an Applied Biosystems ABI 433A peptide synthesiser (standard program Fastmoc 0.1 mmol) using pre-loaded Fmoc-Pro-Trt-TentaGel S resin (455 mg, 0.10 mmol; loading: 0.22 mmol/g). For the coupling reactions, the amino acids Fmoc-Ala-OH, Fmoc-Arg(Pmc)-OH, Fmoc-Asp-OH, Fmoc-Gly-OH, Fmoc-His(Trt)-OH, Fmoc-Pro-OH, Fmoc-Ser(tBu)-OH, Fmoc-Thr(tBu)-OH, and Fmoc-Val-OH were employed. In every coupling cycle, the N-terminal Fmoc group was removed by treatment of the resin with a solution of piperidine (20%) in NMP for at least 3 × 2.5 min. The coupling of the amino acids (1 mmol or 10 equiv based on the loaded resin) was carried out with HBTU (1 mmol), HOBt (1 mmol) and DIPEA (2 mmol) in DMF (20–30 min vortex). After every coupling step, unreacted amino groups were capped by treatment with a mixture of Ac2O (0.5 M), DIPEA (0.125 M) and HOBt (0.015 M) in NMP (10 min vortex). Coupling of the glycosylated β3hThr building block 2a (102.70 mg, 0.15 mmol) was performed using HATU (1.2 equiv with respect to the glycosyl amino acid), HOAt (1.2 equiv) and NMM (4 equiv) for activation (8 h vortex). After coupling of the remaining five amino acids by the standard procedure, the triethylene glycol spacer (1 mmol, 10 equiv based on the loaded resin) was coupled using HBTU (1 mmol), HOBt (1 mmol) and DIPEA (2 mmol) in DMF (20–30 min vortex) and the N-terminal Fmoc group was removed by piperidine (20%) in NMP. Detachment from the resin and simultaneous removal of all side chain protecting groups was performed in a Merrifield glass reactor by shaking with TFA (10 mL), TIS (1.0 mL) and H2O (1.0 mL) for 3 h. The solution was filtered, the resin was washed with CH2Cl2 (5 × 10 mL) and the combined solutions were concentrated in vacuo to a volume of 0.5 mL. After co-evaporation with toluene (3 × 10 mL), the crude product was dissolved in H2O and subjected to lyophilisation. The crude product was purified by RP-HPLC (Luna, MeCN–H2O + 0.1% TFA, 5:95 → 100:0, 50 min, tR= 25.2 min). Yield: 88.3 mg (0.04 mmol), 36%, colourless amorphous solid; [α]D23 = −79.9 (c = 1.00, H2O); 1H NMR (400 MHz, COSY, HSQC, TOCSY, D2O), δ (ppm): 8.50 (m, 1 H, Hε), 7.19 (m, 1 H, Hδ), 5.31–5.28 (m, 1 H, GalNAc-H4), 5.08 (dd, 1 H, J = 3.1 Hz, 11.7 Hz, GalNAc-H3), 5.01 (d, 1 H, J = 3.5 Hz, GalNAc-H1), 4.64–4.54 (m, 2 H, Dα {4.61}, Hα {4.57}), 4.54–4.37 (m, 5 H, Rα {4.52}, A2α {4.47}, A3α {4.46}, A4α {4.45}, S1α {4.39, t, J = 5.5 Hz}), 4.36–4.20 (m, 10 H, S2α {4.33}, hTβ {4.27}, A1α {4.29}, GalNAc-H5 {4.32}, T1α {4.30}, T2α {4.24}, P1–4α {4.29} {4.28} {4.27} {4.24}), 4.19 (d, 1 H, J = 4.5 Hz, Vα), 4.15–3.89 (m, 6 H, GalNAc-H2 {4.12}, T1β, T2β {4.09} {4.07}, GalNAc-H6 {4.02}, P5α {3.94}), 3.89–3.73 (m, 7 H, G1α {3.86}, G2α {3.83}, G1α {3.80}, G2α {3.77}, hTγ {3.77}, S2β {3.76}), 3.73–3.59 (m, 11 H, P1–5δ {3.67}, S1β {3.65}, 2 × CH2-spacer {3.64} {3.62}), 3.59–3.28 (m, 13 H, 2 × CH2-spacer {3.57}, 2 × CH2-spacer {3.54}, P1–5δ {3.52}), 3.18 (dd, 1 H, J = 5.2 Hz, 15.1 Hz, Hβ), 3.11–3.01 (m, 5 H, CH2-spacer {3.08}, Hβ {3.07}, Rδ {3.07}), 2.84 (dd, 1 H, J = 6.5 Hz, 16.8 Hz, Dβ), 2.75 (dd, 1 H, J = 6.5 Hz, 17.3 Hz, Dβ), 2.67–2.40 (m, 4 H, CH2-spacer {2.61} {2.55}, hTα {2.59} {2.46}), 2.23–2.05 (m, 8 H, P1–5β {2.20} {2.17} {2.14}, CH3-Ac {2.07}), 2.01–1.66 (m, 29 H, 4 × CH3-Ac {1.94} {1.92} {1.89} {1.88}, P1–5γ {1.95–1.83}), P1–5β {1.92–1.71}, Vβ {1.86}, Rβ {1.70}), 1.65–1.46 (m, 3 H, Rβ {1.61}, Rγ {1.54}), 1.28–1.17 (m, 12 H, A1–4β {1.24} {1.20}), 1.07–1.01 (m, 9 H, T1γ {1.07}, hTδ {1.06}, T2γ {1.04}), 0.76 (d, 3 H, J = 5.8 Hz, Vγ), 0.71 (d, 3 H, J = 6.4 Hz, Vγ); 13C NMR (101 MHz, DEPT, HSQC, D2O), δ (ppm) = 176.6, 175.7, 174.9, 174.4, 174.3, 173.9, 173.43, 173.4, 173.1, 172.9, 172.8, 172.6, 172.4, 172.3, 171.9, 171.5, 171.3, 171.2, 171.1, 170.9, 170.8, 170.7 (C=O), 163.3, 163.0, 162.6, 162.2 (TFA), 156.7 (C=NH), 133.5 (Hβ), 128.4 (Hε), 117.3 (Hδ), 98.2, 98.0 (GalNAc-C1), 77.2, 76.9 (hTγ), 69.6, 69.5, 69.4, 69.4 (CH2-Spacer), 68.4 (GalNAc-C3), 68.0 (GalNAc-C2), 67.0 (T1β, T2β), 66.3, 66.0 (CH2-spacer), 62.5 (GalNAc-C6), 61.5, 61.1 (S1β, S2β), 60.8, 60.5, 60.0, 59.7, 58.7 (P1–5α), 59.6, 59.3 (T1α, T2α), 58.9 (Vα), 55.5, 54.9, 52.24 (Hα), 51.1 (Rα), 50.5 (hTβ), 50.0 (Dα), 49.6 (GalNAc-C2), 48.0, 47.9, 47.7, 47.4 (P1–5δ), 47.8, 47.8, 47.6, 47.6 (A1α, A2α, A3α, A4α), 42.4, 42.3 (G1α, G2α), 40.5 (CH2-spacer), 39.0 (Rδ), 36.2 (hTα), 34.9 (Dβ), 34.0 (CH2-spacer), 29.9, 29.8 (Vβ), 29.6, 29.3, 29.2, 29.2, 28.1 (P1–5β), 27.4 (Rβ), 26.2 (Hβ), 24.7, 24.6, 24.5, 24.3 (P1–5γ), 23.9 (Rγ), 21.8 (CH3-AcNH), 20.3, 20.0, 20.0 (CH3-Ac) , 18.8, 18.7 (T1γ, T2γ), 18.3, 17.7 (Vγα Vγβ), 16.6 (hTδ), 16.2, 15.4, 15.2, 15.1 (A1–4β); HR-ESI-MS: 1217.0941 ([M+2H]+, calc.: 1217.0932).
Amino-4,7,10-trioxadodecanylamido-N-L-prolyl-L-alanyl-L-histidyl-L-glycyl-L-valyl-O-[α-2-acetamido-2-deoxy-galactopyranosyl]-homo-β3-threonyl-L-seryl-L-alanyl-L-prolyl-L-aspartyl-L-threonyl-L-arginyl-L-prolyl-L-alanyl-L-prolyl-L-glycyl-L-seryl-L-threonyl-L-alanyl-L-proline 8: Peptide 7 was dissolved in 10 mL of methanol (HPLC grade). A fresh solution of sodium methanolate in methanol (0.5 g Na in 25 mL methanol (HPLC grade)) was added drop wise until pH 9.5 was reached. The reaction mixture was stirred over night and neutralised with a few drops of acetic acid. The solvent was removed in vacuo and the residue was dissolved in H2O and subjected to lyophilisation. The crude product was purified by RP-HPLC (Luna, MeCN–H2O + 0.1% TFA, 5:95 → 100:0, 60 min, tR = 15.3 min). Yield: 44 mg (0.02 mmol), 50% (18% based on the loaded resin 5), colourless amorphous solid; [α]D23 = −83.7 (c = 1.00, H2O); 1H NMR (400 MHz, COSY, HSQC, D2O), δ (ppm) = 8.50 (d, 1 H, J = 1.6 Hz, Hδ), 7.20 (d, 1 H, J = 1.3 Hz, Hε), 4.89 (d, 1 H, J = 3.9 Hz, GalNAc-H1), 4.62 (t, 1 H, J = 6.8 Hz, Dα), 4.58 (dd, 1 H, J = 6.7 Hz, 8.6 Hz, Hα), 4.55–4.49 (m, 1 H, Rα), 4.50–4.43 (m, 3 H, A1–3α {4.48} {4.47} {4.45}), 4.40 (t, 1 H, J = 5.4 Hz, S1α), 4.34–4.21 (m, 7 H, S2α {4.32}, P1–5α {4.29} {4.28} {4.28} {4.26} {4.26}, T1α{4.24}), 4.20 (d, 1 H, J = 4.5 Hz, Vα), 4.16–4.10 (m, 1 H, A4α), 4.11–4.05 (m, 2 H, T1β, T2β), 4.00 (dd, 1 H, J = 3.8 Hz, 11.1 Hz, GalNAc-H2), 3.95–3.89 (m, 2 H, T2α {3.93}, GalNAc-H5 {3.89}), 3.89–3.82 (m, 4 H, G1α {3.87}, G1α {3.84}, G2α {3.84}, GalNAc-H4 {3.84}), 3.82–3.74 (m, 3 H, GalNAc-H3 {3.80}, G2α {3.80}, S1β {3.79}), 3.77–3.74 (m, 1 H, S1β), 3.74–3.61 (m, 11 H, P1–5δ {3.70} {3.68} {3.68} {3.68} {3.67}, GalNAc-H6 {3.65}, 2 × CH2-spacer {3.65} {3.62}), 3.61–3.31 (m, 15 H, S2β {3.59}, 4 × CH2-spacer {3.58}, 4 × CH2-spacer {3.54}, P1–5δ {3.58–3.48}), 3.19 (dd, 1 H, J = Hz, Hα), 3.11–3.02 (m, 5 H, CH2-spacer {3.09}, Rδ {3.07}, Hα {3.07}), 2.85 (dd, 1 H, J = 6.6 Hz, 17.4 Hz, Dβ), 2.76 (dd, 1 H, J = 6.6 Hz, 17.0 Hz, Dβ), 2.69–2.38 (m, 4 H, CH2-spacer {2.62}, hTα {2.57}, CH2-spacer{2.55}, hTα {2.43}), 2.26–2.07 (m, 5 H, P1–5β {2.20} {2.18} {2.17} {2.16} {2.14}), 2.00–1.48 (m, 23 H, P1–5γ {1.98–1.80}, P1–5β {1.92–1.70}, CH3-AcNH {1.93}, Vβ {1.85}, Rβ {1.71}, Rβ {1.61}, Rγ {1.54}), 1.29–1.18 (m, 12 H, A1–4β {1.26}, {1.24}, {1.21}, {1.21}), 1.10–1.02 (m, 9 H, T1γ, T2γ, hTδ {1.06}, {1.04}, {1.04}), 0.76 (d, 3 H, J = 6.7 Hz, Vγ), 0.72 (d, 3 H, J = 6.7 Hz, Vγ); 13C NMR (101 MHz, DEPT, HSQC, D2O), δ (ppm) = 175.9, 174.9, 174.4, 174.4, 173.9, 173.5, 173.5, 173.1, 172.9, 172.8, 172.6, 172.5, 172.4, 172.4, 172.0, 171.5, 171.3, 171.2, 171.1, 170.9, 170.8 (C=O), 163.1, 162.7 (TFA), 156.7 (C=NH), 133.4 (Hβ), 128.4 (Hε), 117.3 (Hδ), 98.0 (GalNAc-C1), 76.3 (hTγ), 69.6, 69.5, 69.5, 69.4 (CH2-Spacer), 71.3 (GalNAc-C5), 67.4 (GalNAc-C3), 61.5 (GalNAc-C6), 61.2, 61.1 (S1β, S2β), 60.8, 60.5, 60.0, 59.7, 58.7 (P1–5α), 59.6, 59.3 (T1α, T2α), 58.9 (Vα), 55.5 (S2α), 54.9 (S1α), 52.3 (Hα), 51.1 (Rα), 50.5 (hTβ), 50.1 (Dα), 50.0 (GalNAc-C2), 49.6 (A4α), 48.0, 47.9, 47.7, 47.4 (P1–5δ), 47.8, 47.6, 47.6 (A1α, A2α, A3α), 42.4, 42.3 (G1α, G2α), 40.5 (CH2-spacer), 39.0 (Rδ), 36.1 (hTα), 35.0 (Dβ), 34.0 (CH2-spacer), 29.9 (Vβ), 29.6, 29.3, 29.2, 29.1, 28.7 (P1–5β), 27.4 (Rβ), 26.2 (Hβ), 24.7, 24.6, 24.5, 24.3 (P1–5γ), 23.9 (Rγ), 21.9 (CH3-AcNH), 18.8, 18.7 (T1γ, T2γ), 18.3, 17.7 (Vγα, Vγβ), 16.6 (hTδ), 16.2, 15.4, 15.1, 15.0 (A1–4β); HR-ESI-MS: 1154.0817 ([M+2H]2+, calc.: 1154.0774).
Supporting Information
| Supporting Information File 1: NMR spectra of compounds 2a, 2b, 4, 7, 8 and HPLC chromatogram of compound 2a. | ||
| Format: PDF | Size: 1.9 MB | Download |
References
-
Dwek, R. A. Chem. Rev. 1996, 96, 683–720. doi:10.1021/cr940283b
Return to citation in text: [1] -
Lis, H.; Sharon, N. Eur. J. Biochem. 1993, 218, 1–27. doi:10.1111/j.1432-1033.1993.tb18347.x
Return to citation in text: [1] -
Varki, A. Glycobiology 1993, 3, 97–130. doi:10.1093/glycob/3.2.97
Return to citation in text: [1] -
Pratt, M. R.; Bertozzi, C. R. Chem. Soc. Rev. 2005, 34, 58–68. doi:10.1039/b400593g
Return to citation in text: [1] -
Taylor-Papadimitriou, J.; Epenetos, A. A. Trends Biotechnol. 1994, 12, 227–233. doi:10.1016/0167-7799(94)90121-X
Return to citation in text: [1] -
Danishefsky, S. J.; Allen, J. R. Angew. Chem., Int. Ed. 2000, 39, 836–863. doi:10.1002/(SICI)1521-3773(20000303)39:5<836::AID-ANIE836>3.0.CO;2-I
Return to citation in text: [1] -
Slovin, S. S.; Keding, S. J.; Ragupathi, G. Immunol. Cell Biol. 2005, 83, 418–428. doi:10.1111/j.1440-1711.2005.01350.x
Return to citation in text: [1] -
Becker, T.; Dziadek, S.; Wittrock, S.; Kunz, H. Curr. Opin. Mol. Ther. 2007, 9, 35–44. doi:10.2174/156800906778194577
Return to citation in text: [1] -
Liakatos, A.; Kunz, H. Curr. Opin. Mol. Ther. 2007, 9, 35–44.
Return to citation in text: [1] -
Buskas, T.; Thompson, P.; Boons, G.-J. Chem. Commun. 2009, 5335–5349. doi:10.1039/b908664c
Return to citation in text: [1] -
Urban, D.; Skrydstrup, T.; Beau, J.-M. Chem. Commun. 1998, 955–956. doi:10.1039/a801196f
Return to citation in text: [1] -
Röhrig, C. H.; Takhi, M.; Schmidt, R. R. Synlett 2001, 1170–1172. doi:10.1055/s-2001-15144
Return to citation in text: [1] -
Kuberan, B.; Sikkander, S. A.; Tomiyama, H.; Linhardt, R. J. Angew. Chem., Int. Ed. 2003, 42, 2073–2075. doi:10.1002/anie.200351099
Return to citation in text: [1] -
Cipolla, L.; Rescigno, M.; Leone, A.; Peri, F.; La Ferla, B.; Nicotra, F. Bioorg. Med. Chem. 2002, 10, 1639–1646. doi:10.1016/S0968-0896(01)00433-3
Return to citation in text: [1] -
Rich, J. J.; Bundle, D. R. Org. Lett. 2004, 6, 897–900. doi:10.1021/ol036460p
Return to citation in text: [1] -
Bousquet, E.; Spadaro, A.; Pappalardo, M. S.; Bernardini, R.; Romeo, R.; Panza, L.; Ronsisvalle, G. J. Carbohydr. Chem. 2000, 19, 527–541. doi:10.1080/07328300008544097
Return to citation in text: [1] -
Bundle, D. R.; Rich, J. J.; Jacques, S.; Yu, H. N.; Nitz, M.; Ling, C.-C. Angew. Chem., Int. Ed. 2005, 44, 7725–7729. doi:10.1002/anie.200502179
Return to citation in text: [1] -
Rich, J. J.; Wakarchuk, W. W.; Bundle, D. R. Chem.–Eur. J. 2006, 12, 845–858. doi:10.1002/chem.200500518
Return to citation in text: [1] -
Wu, X.; Lipinski, T.; Paszkiewicz, E.; Bundle, D. R. Chem.–Eur. J. 2008, 14, 6474–6482. doi:10.1002/chem.200800352
Return to citation in text: [1] -
Mersch, C.; Wagner, S.; Hoffmann-Röder, A. Synlett 2009, 2167–2171. doi:10.1055/s-0029-1217566
Return to citation in text: [1] -
Hook, D. F.; Bindschädler, P.; Mahajan, Y. R.; Šebesta, R.; Kast, P.; Seebach, D. Chem. Biodiversity 2005, 2, 591–632. doi:10.1002/cbdv.200590039
Return to citation in text: [1] -
Aguilar, M.-I.; Purcell, A. W.; Devi, R.; Lew, R.; Rossjohn, J.; Smith, A. I.; Perlmutter, P. Org. Biomol. Chem. 2007, 5, 2884–2890. doi:10.1039/b708507a
Return to citation in text: [1] [2] -
Cheng, R. P.; Gellman, S. H.; DeGrado, W. F. Chem. Rev. 2001, 101, 3219–3232. doi:10.1021/cr000045i
Return to citation in text: [1] -
Steer, D. L.; Lew, R. A.; Perlmutter, P.; Smith, A. I.; Aguilar, M.-I. Curr. Med. Chem. 2002, 9, 811–822.
Return to citation in text: [1] -
Seebach, D.; Gardiner, J. Acc. Chem. Res. 2008, 41, 1366–1375. doi:10.1021/ar700263g
Return to citation in text: [1] -
Saludes, J. P.; Ames, J. B.; Gervay-Hague, J. J. Am. Chem. Soc. 2009, 131, 5495–5505. doi:10.1021/ja808286x
Return to citation in text: [1] -
Disney, M. D.; Hook, D. F.; Namoto, K.; Seeberger, P. H.; Seebach, D. Chem. Biodiversity 2005, 2, 1624–1634. doi:10.1002/cbdv.200590132
Return to citation in text: [1] -
Norgren, A. S.; Geitmann, M.; Danielson, U. H.; Arvidsson, P. I. J. Mol. Recognit. 2007, 20, 132–138. doi:10.1002/jmr.821
Return to citation in text: [1] -
Norgren, A. S.; Norberg, T.; Arvidsson, P. I. J. Pept. Sci. 2007, 13, 717–727. doi:10.1002/psc.832
Return to citation in text: [1] [2] [3] -
Norgren, A. S.; Arvidsson, P. I. Org. Biomol. Chem. 2005, 3, 1359–1361. doi:10.1039/b503237g
Return to citation in text: [1] -
Norgren, A. S.; Arvidsson, P. I. J. Org. Chem. 2008, 73, 5272–5278. doi:10.1021/jo8003265
Return to citation in text: [1] -
Paulsen, H.; Hölck, J.-P. Carbohydr. Res. 1982, 109, 89–107. doi:10.1016/0008-6215(82)84033-0
Return to citation in text: [1] [2] [3] -
Brocke, C.; Kunz, H. Synthesis 2004, 525–542. doi:10.1055/s-2004-815962
Return to citation in text: [1] [2] [3] -
Dziadek, S.; Brocke, C.; Kunz, H. Chem.–Eur. J. 2004, 10, 4150–4162. doi:10.1002/chem.200400228
Return to citation in text: [1] -
Dziadek, S.; Kowalczyk, D.; Kunz, H. Angew. Chem., Int. Ed. 2005, 44, 7624–7630. doi:10.1002/anie.200501593
Return to citation in text: [1] -
Kaiser, A.; Gaidzik, N.; Westerlind, U.; Kowalczyk, D.; Hobel, A.; Schmitt, E.; Kunz, H. Angew. Chem., Int. Ed. 2009, 48, 7551–7555. doi:10.1002/anie.200902564
Return to citation in text: [1] -
Westerlind, U.; Schröder, H.; Hobel, A.; Gaidzik, N.; Kaiser, A.; Niemeyer, C. M.; Schmitt, E.; Waldmann, H.; Kunz, H. Angew. Chem., Int. Ed. 2009, 48, 8263–8267. doi:10.1002/anie.200902963
Return to citation in text: [1] -
Fréchet, J. M. J.; Haque, K. E. Tetrahedron Lett. 1975, 16, 3055–3056. doi:10.1016/S0040-4039(00)75071-4
Return to citation in text: [1] -
Dourtoglou, V.; Gross, B.; Lambropoulou, V.; Zioudrou, C. Synthesis 1984, 572–574. doi:10.1055/s-1984-30895
Return to citation in text: [1] -
Carpino, L. A. J. Am. Chem. Soc. 1993, 115, 4397–4398. doi:10.1021/ja00063a082
Return to citation in text: [1] -
Keil, S.; Claus, C.; Dippold, W.; Kunz, H. Angew. Chem., Int. Ed. 2001, 40, 366–369. doi:10.1002/1521-3773(20010119)40:2<366::AID-ANIE366>3.0.CO;2-J
Return to citation in text: [1] -
Lombardi, P. Chem. Ind. (London) 1994, 708.
Return to citation in text: [1]
| 1. | Dwek, R. A. Chem. Rev. 1996, 96, 683–720. doi:10.1021/cr940283b |
| 2. | Lis, H.; Sharon, N. Eur. J. Biochem. 1993, 218, 1–27. doi:10.1111/j.1432-1033.1993.tb18347.x |
| 3. | Varki, A. Glycobiology 1993, 3, 97–130. doi:10.1093/glycob/3.2.97 |
| 11. | Urban, D.; Skrydstrup, T.; Beau, J.-M. Chem. Commun. 1998, 955–956. doi:10.1039/a801196f |
| 12. | Röhrig, C. H.; Takhi, M.; Schmidt, R. R. Synlett 2001, 1170–1172. doi:10.1055/s-2001-15144 |
| 13. | Kuberan, B.; Sikkander, S. A.; Tomiyama, H.; Linhardt, R. J. Angew. Chem., Int. Ed. 2003, 42, 2073–2075. doi:10.1002/anie.200351099 |
| 14. | Cipolla, L.; Rescigno, M.; Leone, A.; Peri, F.; La Ferla, B.; Nicotra, F. Bioorg. Med. Chem. 2002, 10, 1639–1646. doi:10.1016/S0968-0896(01)00433-3 |
| 32. | Paulsen, H.; Hölck, J.-P. Carbohydr. Res. 1982, 109, 89–107. doi:10.1016/0008-6215(82)84033-0 |
| 33. | Brocke, C.; Kunz, H. Synthesis 2004, 525–542. doi:10.1055/s-2004-815962 |
| 6. | Danishefsky, S. J.; Allen, J. R. Angew. Chem., Int. Ed. 2000, 39, 836–863. doi:10.1002/(SICI)1521-3773(20000303)39:5<836::AID-ANIE836>3.0.CO;2-I |
| 7. | Slovin, S. S.; Keding, S. J.; Ragupathi, G. Immunol. Cell Biol. 2005, 83, 418–428. doi:10.1111/j.1440-1711.2005.01350.x |
| 8. | Becker, T.; Dziadek, S.; Wittrock, S.; Kunz, H. Curr. Opin. Mol. Ther. 2007, 9, 35–44. doi:10.2174/156800906778194577 |
| 9. | Liakatos, A.; Kunz, H. Curr. Opin. Mol. Ther. 2007, 9, 35–44. |
| 10. | Buskas, T.; Thompson, P.; Boons, G.-J. Chem. Commun. 2009, 5335–5349. doi:10.1039/b908664c |
| 32. | Paulsen, H.; Hölck, J.-P. Carbohydr. Res. 1982, 109, 89–107. doi:10.1016/0008-6215(82)84033-0 |
| 5. | Taylor-Papadimitriou, J.; Epenetos, A. A. Trends Biotechnol. 1994, 12, 227–233. doi:10.1016/0167-7799(94)90121-X |
| 29. | Norgren, A. S.; Norberg, T.; Arvidsson, P. I. J. Pept. Sci. 2007, 13, 717–727. doi:10.1002/psc.832 |
| 4. | Pratt, M. R.; Bertozzi, C. R. Chem. Soc. Rev. 2005, 34, 58–68. doi:10.1039/b400593g |
| 29. | Norgren, A. S.; Norberg, T.; Arvidsson, P. I. J. Pept. Sci. 2007, 13, 717–727. doi:10.1002/psc.832 |
| 22. | Aguilar, M.-I.; Purcell, A. W.; Devi, R.; Lew, R.; Rossjohn, J.; Smith, A. I.; Perlmutter, P. Org. Biomol. Chem. 2007, 5, 2884–2890. doi:10.1039/b708507a |
| 23. | Cheng, R. P.; Gellman, S. H.; DeGrado, W. F. Chem. Rev. 2001, 101, 3219–3232. doi:10.1021/cr000045i |
| 24. | Steer, D. L.; Lew, R. A.; Perlmutter, P.; Smith, A. I.; Aguilar, M.-I. Curr. Med. Chem. 2002, 9, 811–822. |
| 25. | Seebach, D.; Gardiner, J. Acc. Chem. Res. 2008, 41, 1366–1375. doi:10.1021/ar700263g |
| 27. | Disney, M. D.; Hook, D. F.; Namoto, K.; Seeberger, P. H.; Seebach, D. Chem. Biodiversity 2005, 2, 1624–1634. doi:10.1002/cbdv.200590132 |
| 28. | Norgren, A. S.; Geitmann, M.; Danielson, U. H.; Arvidsson, P. I. J. Mol. Recognit. 2007, 20, 132–138. doi:10.1002/jmr.821 |
| 29. | Norgren, A. S.; Norberg, T.; Arvidsson, P. I. J. Pept. Sci. 2007, 13, 717–727. doi:10.1002/psc.832 |
| 21. | Hook, D. F.; Bindschädler, P.; Mahajan, Y. R.; Šebesta, R.; Kast, P.; Seebach, D. Chem. Biodiversity 2005, 2, 591–632. doi:10.1002/cbdv.200590039 |
| 22. | Aguilar, M.-I.; Purcell, A. W.; Devi, R.; Lew, R.; Rossjohn, J.; Smith, A. I.; Perlmutter, P. Org. Biomol. Chem. 2007, 5, 2884–2890. doi:10.1039/b708507a |
| 30. | Norgren, A. S.; Arvidsson, P. I. Org. Biomol. Chem. 2005, 3, 1359–1361. doi:10.1039/b503237g |
| 31. | Norgren, A. S.; Arvidsson, P. I. J. Org. Chem. 2008, 73, 5272–5278. doi:10.1021/jo8003265 |
| 20. | Mersch, C.; Wagner, S.; Hoffmann-Röder, A. Synlett 2009, 2167–2171. doi:10.1055/s-0029-1217566 |
| 15. | Rich, J. J.; Bundle, D. R. Org. Lett. 2004, 6, 897–900. doi:10.1021/ol036460p |
| 16. | Bousquet, E.; Spadaro, A.; Pappalardo, M. S.; Bernardini, R.; Romeo, R.; Panza, L.; Ronsisvalle, G. J. Carbohydr. Chem. 2000, 19, 527–541. doi:10.1080/07328300008544097 |
| 17. | Bundle, D. R.; Rich, J. J.; Jacques, S.; Yu, H. N.; Nitz, M.; Ling, C.-C. Angew. Chem., Int. Ed. 2005, 44, 7725–7729. doi:10.1002/anie.200502179 |
| 18. | Rich, J. J.; Wakarchuk, W. W.; Bundle, D. R. Chem.–Eur. J. 2006, 12, 845–858. doi:10.1002/chem.200500518 |
| 19. | Wu, X.; Lipinski, T.; Paszkiewicz, E.; Bundle, D. R. Chem.–Eur. J. 2008, 14, 6474–6482. doi:10.1002/chem.200800352 |
| 26. | Saludes, J. P.; Ames, J. B.; Gervay-Hague, J. J. Am. Chem. Soc. 2009, 131, 5495–5505. doi:10.1021/ja808286x |
| 35. | Dziadek, S.; Kowalczyk, D.; Kunz, H. Angew. Chem., Int. Ed. 2005, 44, 7624–7630. doi:10.1002/anie.200501593 |
| 33. | Brocke, C.; Kunz, H. Synthesis 2004, 525–542. doi:10.1055/s-2004-815962 |
| 34. | Dziadek, S.; Brocke, C.; Kunz, H. Chem.–Eur. J. 2004, 10, 4150–4162. doi:10.1002/chem.200400228 |
| 32. | Paulsen, H.; Hölck, J.-P. Carbohydr. Res. 1982, 109, 89–107. doi:10.1016/0008-6215(82)84033-0 |
| 33. | Brocke, C.; Kunz, H. Synthesis 2004, 525–542. doi:10.1055/s-2004-815962 |
| 40. | Carpino, L. A. J. Am. Chem. Soc. 1993, 115, 4397–4398. doi:10.1021/ja00063a082 |
| 41. | Keil, S.; Claus, C.; Dippold, W.; Kunz, H. Angew. Chem., Int. Ed. 2001, 40, 366–369. doi:10.1002/1521-3773(20010119)40:2<366::AID-ANIE366>3.0.CO;2-J |
| 38. | Fréchet, J. M. J.; Haque, K. E. Tetrahedron Lett. 1975, 16, 3055–3056. doi:10.1016/S0040-4039(00)75071-4 |
| 39. | Dourtoglou, V.; Gross, B.; Lambropoulou, V.; Zioudrou, C. Synthesis 1984, 572–574. doi:10.1055/s-1984-30895 |
| 36. | Kaiser, A.; Gaidzik, N.; Westerlind, U.; Kowalczyk, D.; Hobel, A.; Schmitt, E.; Kunz, H. Angew. Chem., Int. Ed. 2009, 48, 7551–7555. doi:10.1002/anie.200902564 |
| 37. | Westerlind, U.; Schröder, H.; Hobel, A.; Gaidzik, N.; Kaiser, A.; Niemeyer, C. M.; Schmitt, E.; Waldmann, H.; Kunz, H. Angew. Chem., Int. Ed. 2009, 48, 8263–8267. doi:10.1002/anie.200902963 |
© 2010 Karch and Hoffmann-Röder; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)