Abstract
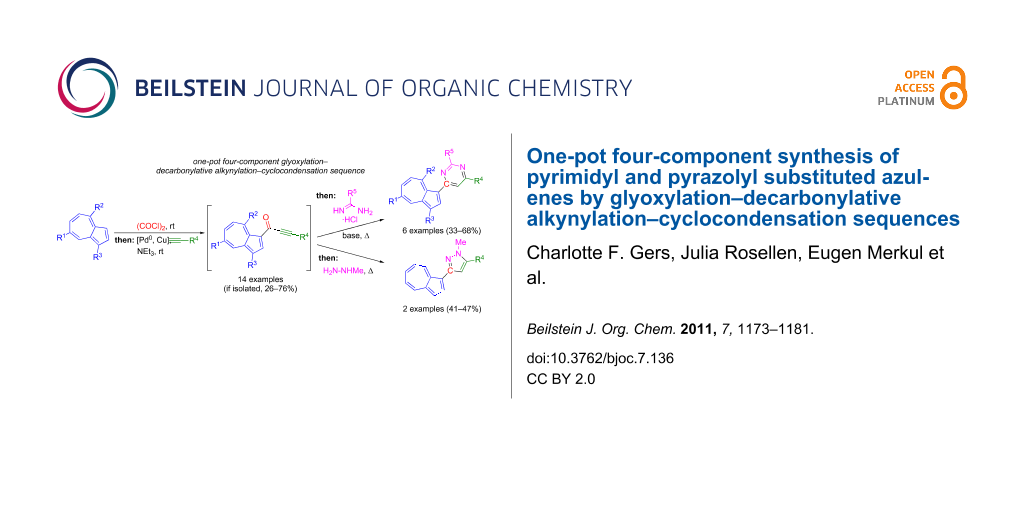
A novel one-pot four-component synthesis of pyrimidyl- and pyrazolylazulenes through the use of glyoxylation–decarbonylative alkynylation–cyclocondensation sequences starting from azulene or guaiazulene as substrates, gives rise to the formation of the target compounds in moderate to good yields.
Graphical Abstract
Introduction
Diversity-oriented synthesis has become an important field in organic chemistry, initiated by the increasing demand for new scaffolds for pharmaceuticals and biologically active compounds over the past decades [1-3]. Herein, multicomponent reactions adopt a central position since each component can be varied within a wide range of functionalities and substituents [4-8]. Furthermore, these one-pot processes are highly advantageous because they combine shortened reaction times and resource efficiency with diminished waste production in comparison to traditional multistep syntheses. Thus, they can be considered to be economically and ecologically efficient [9,10].
In particular, multicomponent syntheses of heterocycles initiated by transition metal catalysis received increasing attention in the past decade [11]. As a one-pot synthetic methodology, this novel concept combines the unique reactivity patterns of transition metal catalysis with fundamental organic reactivity, in a sequential or consecutive fashion. Over the years, we have contributed to this concept through Pd/Cu-catalyzed accesses to enones and ynones and the in situ transformation of these intermediates into many classes of heterocycles [12-15]. These novel MCRs nicely correspond with diversity-oriented strategies towards functional organic chromophores [1,2].
The striking blue color of azulene (1a) (from the Spanish word “azul” = blue) has aroused scientific attention for a long time [16,17]. This prominent appearance results from the electronic transition between the S0 and S1 state [18], as a consequence of low energy frontier molecular orbital transitions [19]. The bicyclic structure of this nonbenzoid hydrocarbon results from a five–seven ring annulation with a planar, cyclic conjugation of 10 π-electrons. The dipole moment of 1a at μ = 1.08 D [20] is astoundingly large in comparison to that of naphthalene at μ = 0 D and can be rationalized by a significant contribution of cyclopentadienyl anion/tropylium cation resonance structures (Scheme 1) [19].
Scheme 1: Selected resonance structures of azulene (1a) and structure of the sesquiterpene guaiazulene (1b).
Scheme 1: Selected resonance structures of azulene (1a) and structure of the sesquiterpene guaiazulene (1b).
Since the elucidation of the structure and the first synthesis of the azulene skeleton by Pfau and Plattner [21,22], its reactivity has been intensively studied [23-26]. The aromatic system is susceptible to nucleophilic addition in the 4-, 6- and 8-positions [23], whereas electrophilic aromatic substitution, such as Friedel–Crafts-type reactions, generally occurs in the 1-position [24]. Interestingly, the azulene motif is also found in terpenoids [27,28]. Guaiazulene (1b) (Scheme 1), a commonly known derivative of azulene (1a), is a naturally occurring sesquiterpene [29]. Guaiazulene (1b) has found entry in a wide range of cosmetic formulations [30]. In addition, numerous azulene derivatives display appealing properties for material [31-33] and pharmaceutical sciences [34-38]. Furthermore, the use of the azulene moiety as part of a protecting group chromophore in carbohydrate chemistry has recently been reported [39].
N-Heteroaryl-substituted azulenes can be accessed by stoichiometric [40,41] as well as Pd-catalyzed cross-coupling processes [42-44]. However, these methods have only delivered a narrow range of derivatives. Prior to application in Pd-catalyzed processes, azulenes must be functionalized, either by halogenation or borylation, and some of these derivatives were found to be quite unstable [45,46]. To the best of our knowledge, no diversity-oriented multicomponent syntheses of azulenyl heterocycles have been reported so far. Here, we report the development of one-pot four-component syntheses toward pyrimidyl- and pyrazolylazulenes.
Results and Discussion
Recently, we reported a three-component synthesis leading to the formation of ynones by a conceptually novel glyoxylation–decarbonylative Sonogashira coupling sequence (Scheme 2) [47]. The Lewis acid free glyoxylation of electron rich N-heterocycles, such as indoles and pyrroles, leads to the formation of glyoxylyl chlorides, which can be reacted without isolation by decarbonylative Sonogashira coupling to form the desired ynones. So far, only one example of the synthesis of azulenylynones has been described [48].
Scheme 2: Synthesis of ynones by glyoxylation–decarbonylative Sonogashira coupling.
Scheme 2: Synthesis of ynones by glyoxylation–decarbonylative Sonogashira coupling.
Our retrosynthetic analysis (Scheme 3) suggests that a wide range of N-heterocycle-substituted azulenes should be accessible through Michael addition–cyclocondensation of azulenylynones with binucleophiles. Azulenylynones in turn could be simply disconnected by our glyoxylation–decarbonylative alkynylation transform [47] back to azulenes.
Scheme 3: Retrosynthetic analysis of N-heterocyclic substituted azulenes by a one-pot four-component approach.
Scheme 3: Retrosynthetic analysis of N-heterocyclic substituted azulenes by a one-pot four-component approach....
Previously, glyoxylation of azulene (1a), with oxalyl chloride in 1-position was reported to be essentially complete within 5 min [39]. Oxalyl bromide could be equally used as a glyoxylating agent [49,50]. Likewise, the glyoxylation of 1b has been reported to proceed in 3-position with both reagents, yet with lower reactivity, and its conversion was found to be incomplete even after 2 h. In addition, the formation of side products [51] and decarbonylation [52] was observed, presumably caused by the steric hindrance of the methyl group in 4-position.
Encouraged by our smooth glyoxylation–alkynylation sequences with a variety of unfunctionalized π-nucleophiles, such as pyrazoles, thiophenes, furans, and even the hydrocarbon azulene (1a) [53], we decided to perform optimization studies of the glyoxylation–decarbonylative alkynylation with guaiazulene (1b), a commercially available and inexpensive azulene derivative, and 1-hexyne (2b) as model substrates (Table 1) (for experimental details, see Supporting Information File 1).
Table 1: Optimization studies for the synthesis of ynone 3k.a
|
|
|||||||||
| Glyoxylation step | Sonogashira coupling step | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | NEt3 [equiv] | T [°C] | t [h] | PdCl2(PPh3)2 [mol %] | CuI [mol %] | NEt3 [equiv] | t [h] | Yield 3k [%]b | |
| 1 | 1.0 | 0 °C to rt | 4 | 1 | 1 | 1.0 | 1 | 40 | |
| 2 | - | 0 °C to rt | 4 | 1 | 1 | 2.0 | 1 | 43 | |
| 3 | 1.0 | 0 °C to rt | 24 | 1 | 1 | 1.0 | 1 | 36 | |
| 4 | 1.0 | 0 °C to rt | 2 | 1 | 1 | 1.0 | 1 | 25 | |
| 5 | 1.0 | 0 °C to rt | 4 | 1 | 1 | 1.0 | 2 | 41 | |
| 6c | - | rt to 50 °C | 4 | 1 | 1 | 2.0 | 1 | 19 | |
| 7 | - | 0 °C to rt | 4 | 2 | 2 | 2.0 | 1 | 56 | |
| 8c | - | rt | 4 | 2 | 2 | 2.0 | 1 | 55 | |
aThe reactions were performed on a 2.00 mmol scale in 10 mL of THF as a solvent (c (1b) = 0.2 M); bisolated yield; c1,4-Dioxane was used as a solvent (c (1b) = 0.2 M).
Initially, the optimized conditions for the glyoxylation–decarbonylative alkynylation of indoles were applied [47], except for the addition of one equivalent of triethylamine in the glyoxylation step for scavenging the generated hydrogen chloride (Table 1, entry 1). However, the use of the amine base in the first step was unsatisfactory (Table 1, entry 2). Prolonged reaction times in the first step did not affect the yield. According to monitoring by TLC, glyoxylation of guaiazulene (1b) was incomplete even after 24 h reaction time (Table 1, entry 3). Shorter reaction times in the first step caused a substantial decrease of the yield (Table 1, entry 4), whereas longer reaction times in the Sonogashira coupling had no effect on the yield (Table 1, entry 5). Rising the reaction temperature of the glyoxylation step to 50 °C considerably diminished the yield (Table 1, entry 6). However, doubling the catalyst loading furnished significantly higher yields (Table 1, entry 7). 1,4-Dioxane was equally well employed as a solvent (Table 1, entry 8). From this optimization study, the conditions of entry 7 (Table 1) were considered to be optimal and were applied in the three-component synthesis of the azulenylynones 3 (Scheme 4, Table 2) (for experimental details, see Supporting Information File 1). Their structures were unambiguously supported by NMR spectroscopy, mass spectrometry, and combustion analysis.
Scheme 4: Three-component synthesis of azulenyl- and guaiazulenylynones 3 by glyoxylation–decarbonylative Sonogashira coupling sequence.
Scheme 4: Three-component synthesis of azulenyl- and guaiazulenylynones 3 by glyoxylation–decarbonylative Son...
Table 2: Three-component synthesis of azulenyl- and guaiazulenylynones 3.a
| Entry | Azulene 1 | Alkyne 2 | Azulenylynone 3 | [%]b |
|---|---|---|---|---|
| 1 | 1a (R1 = R2 = R3 = H) | 2a (R4 = Ph) |
3a |
65c |
| 2 | 1a | 2b (R4 = n-Bu) |
3b |
66c |
| 3 | 1b (R1 = iPr, R2 = R3 = Me) | 2a |
3c |
55 |
| 4 | 1b | 2c (R4 = p-tolyl) |
3d |
57 |
| 5 | 1b | 2d (R4 = p-CNC6H4) |
3e |
60 |
| 6 | 1b | 2e (R4 = p-NO2C6H4) |
3f |
76 |
| 7 | 1b | 2f (R4 = m-FC6H4) |
3g |
51 |
| 8 | 1b | 2g (R4 = 3,5-(MeO)2C6H3) |
3h |
47 |
| 9 | 1b | 2h (R4 = 2-C4H3S) |
3i |
55 |
| 10 | 1b | 2i (R4 = 3-pyridyl) |
3j |
31 |
| 11 | 1b | 2b |
3k |
56 |
| 12 | 1b | 2j (R4 = cyclopropyl) |
3l |
42 |
| 13 | 1b | 2k (R4 = CH(OEt)2) |
3m |
30 |
| 14 | 1b | 2l (R4 = TIPS) |
3n |
26 |
aThe reactions were performed on a 2.00 mmol scale in 10 mL of THF as a solvent (c (1) = 0.2 M); bIsolated and purified compounds; cThe reactions were performed on a 1.00 mmol scale in 5 mL THF as a solvent (c (1) = 0.2 M).
Azulene (1a) and guaiazulene (1b) were both applied as substrates in the reaction sequence, giving rise to azulenyl- and guaiazulenylynones 3. The azulenyl derivatives 3a and 3b were obtained in higher yields compared to the guaiazulenylynones 3c–n. A variety of substituted arylacetylenes were utilized in the reaction sequence. Electron neutral (Table 2, entries 1 and 3), electron withdrawing (Table 2, entries 5–7), and electron donating (Table 2, entries 4 and 8) substituents were equally well tolerated. In addition, heteroaryl-substituted acetylenes (Table 2, entries 9 and 10) as well as simple aliphatic acetylenes (Table 2, entries 2, 11, and 12) were successfully employed. Finally, propargylaldehyde diethylacetal (Table 2, entry 13) and TIPS-protected acetylene (Table 2, entry 14) also participated in the sequence, although relatively low yields were achieved.
With this versatile three-component synthesis of azulenylynones in hand, the stage was set to expand the sequence to a four-component access to pyrimidyl- and pyrazolyl-substituted azulenes. Hence, the conditions for the terminating Michael addition–cyclocondensation step, adopted from a recent work [54], were only slightly adjusted as a consequence of the lower electrophilicity of azulenylynones in comparison to aryl- and heteroaryl-substituted ynones that we have previously synthesized. Therefore, upon the subsequent reaction of the azulenes 1 with oxalyl chloride, followed by Pd/Cu-catalyzed decarbonylative alkynylation with terminal alkynes 2, and finally by cyclocondensation of the ynone intermediates with substituted amidine hydrochlorides 4, pyrimidylazulenes 5 were obtained in moderate to good yields in a one-pot fashion (Scheme 5) (for experimental details, see Supporting Information File 1).
Scheme 5: Four-component synthesis of pyrimidylazulenes 5 by glyoxylation–decarbonylative Sonogashira coupling–cyclocondensation sequence (yields refer to isolated and purified compounds).
Scheme 5: Four-component synthesis of pyrimidylazulenes 5 by glyoxylation–decarbonylative Sonogashira couplin...
The diversity-oriented nature of this four-component approach to pyrimidylazulenes 5 is underlined by flexible variation of the azulenyl, the alkynyl, and the amidinyl substrates. In particular, the amidine component 4 leads to the formation of aryl (compounds 5a, 5d–5f), heteroaryl (compound 5b) or alkyl (compound 5c) pyrimidylazulene derivatives.
Likewise, pyrazolylazulenes were obtained in the course of a consecutive glyoxylation–decarbonylative Sonogashira coupling, followed by a cyclocondensation with methylhydrazine (6) to furnish two N-methylpyrazoles 7 in moderate yields (Scheme 6) (for experimental details, see Supporting Information File 1).
Scheme 6: Four-component synthesis of pyrazolylazulenes 7 by glyoxylation–decarbonylative Sonogashira coupling–cyclocondensation sequence (yields refer to isolated and purified compounds).
Scheme 6: Four-component synthesis of pyrazolylazulenes 7 by glyoxylation–decarbonylative Sonogashira couplin...
Attempts to employ phenylhydrazine, N-Boc-hydrazine, and hydrazine hydrate under standard conditions were met with failure. Based upon previous syntheses of N-methylpyrazoles from ynones and methylhydrazine [55,56] and the appearance of a single set of resonances in the proton and carbon NMR spectra, it is obvious that only a single regioisomer was formed. Although the synthesis of similarly substituted pyrazolylazulenes has already been described [57], our one-pot four-component approach utilizes readily available starting materials as well as a simple catalyst system. In addition, it avoids tedious multiple workup and purification operations.
Conclusion
In conclusion, we have developed a one-pot four-component process for the synthesis of novel pyrimidyl- and pyrazolylazulenes. A wide range of substituents can be introduced by this modular approach to N-heterocyclic azulene derivatives. The key step of this diversity-oriented synthesis is the generation of azulenylynones by the glyoxylation–decarbonylative alkynylation sequence with azulene or guaiazulene as substrates. Undoubtedly, this novel four-component approach to heterocyclic derivatives of azulene is well suited for the development of functional chromophores with extended π-conjugation.
Supporting Information
| Supporting Information File 1: Experimental procedures, spectroscopic and analytical data, and copies of NMR spectra of compounds 3, 5, and 7. | ||
| Format: PDF | Size: 1.5 MB | Download |
References
-
Müller, T. J. J.; D’Souza, D. M. Pure Appl. Chem. 2008, 80, 609–620. doi:10.1351/pac200880030609
Return to citation in text: [1] [2] -
Müller, T. J. J. In Functional Organic Materials. Synthesis, Strategies, and Applications; Müller, T. J. J.; Bunz, U. H. F., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp 179–223.
Return to citation in text: [1] [2] -
Burke, M. D.; Schreiber, S. L. Angew. Chem., Int. Ed. 2004, 43, 46–58. doi:10.1002/anie.200300626
Return to citation in text: [1] -
Sunderhaus, J. D.; Martin, S. F. Chem.–Eur. J. 2009, 15, 1300–1308. doi:10.1002/chem.200802140
Return to citation in text: [1] -
Isambert, N.; Lavilla, R. Chem.–Eur. J. 2008, 14, 8444–8454. doi:10.1002/chem.200800473
Return to citation in text: [1] -
Orru, R. V. A.; de Greef, M. Synthesis 2003, 1471–1499. doi:10.1055/s-2003-40507
Return to citation in text: [1] -
Touré, B. B.; Hall, D. G. Chem. Rev. 2009, 109, 4439–4486. doi:10.1021/cr800296p
Return to citation in text: [1] -
Bonne, D.; Coquerel, Y.; Constantieux, T.; Rodriguez, J. Tetrahedron: Asymmetry 2010, 21, 1085–1109. doi:10.1016/j.tetasy.2010.04.045
Return to citation in text: [1] -
Tietze, L. F. Chem. Rev. 1996, 96, 115–136. doi:10.1021/cr950027e
Return to citation in text: [1] -
Coquerel, Y.; Boddaert, T.; Presset, M.; Mailhol, D.; Rodriguez, J. Multiple Bond-Forming Transformations: The Key Concept toward Eco-Compatible Synthetic Organic Chemistry. In Ideas in Chemistry and Molecular Sciences: Advances in Synthetic Chemistry; Pignataro, B., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp 187–202. doi:10.1002/9783527630554.ch9
Return to citation in text: [1] -
D’Souza, D. M.; Müller, T. J. J. Chem. Soc. Rev. 2007, 36, 1095–1108. doi:10.1039/B608235C
Return to citation in text: [1] -
Müller, T. J. J. Palladium-Copper Catalyzed Alkyne Activation as an Entry to Multicomponent Syntheses of Heterocycles. In Synthesis of Heterocycles via Multicomponent Reactions II; Orru, R. V. A.; Ruijter, E., Eds.; Topics in Heterocyclic Chemistry, Vol. 25; Springer: Berlin/Heidelberg, Germany, 2010; pp 25–94. doi:10.1007/7081_2010_43
Return to citation in text: [1] -
Willy, B.; Müller, T. J. J. Curr. Org. Chem. 2009, 13, 1777–1790. doi:10.2174/138527209789630479
Return to citation in text: [1] -
Willy, B.; Müller, T. J. J. ARKIVOC 2008, 2008 (i), 195–208.
Return to citation in text: [1] -
Müller, T. J. J. Multi-component syntheses of heterocycles by virtue of palladium catalyzed generation of alkynones and chalcones. In Targets in Heterocyclic Systems; Attanasi, O.; Spinelli, D., Eds.; Italian Society of Chemistry: Rome, Italy, 2006; Vol. 10, pp 54–65.
Return to citation in text: [1] -
Hansen, H. J. Chimia 1996, 50, 489–496.
Return to citation in text: [1] -
Hansen, H. J. Chimia 1997, 51, 147–159.
Return to citation in text: [1] -
Liu, R. S. H. J. Chem. Educ. 2002, 79, 183–185. doi:10.1021/ed079p183
Return to citation in text: [1] -
Zeller, K.-P. Houben-Weyl; 1985; Vol. 5/2c, pp 127–133.
and references cited therein.
Return to citation in text: [1] [2] -
Anderson, A. G., Jr.; Steckler, B. M. J. Am. Chem. Soc. 1959, 81, 4941–4946. doi:10.1021/ja01527a046
Return to citation in text: [1] -
Pfau, A. S.; Plattner, P. A. Helv. Chim. Acta 1936, 19, 858–879. doi:10.1002/hlca.193601901117
Return to citation in text: [1] -
Plattner, P. A.; Pfau, A. S. Helv. Chim. Acta 1937, 20, 224–232. doi:10.1002/hlca.19370200133
Return to citation in text: [1] -
Hafner, K.; Bernhard, C.; Müller, R. Justus Liebigs Ann. Chem. 1961, 650, 35–41. doi:10.1002/jlac.19616500104
Return to citation in text: [1] [2] -
Anderson, A. G., Jr.; Nelson, J. A. J. Am. Chem. Soc. 1950, 72, 3824–3825. doi:10.1021/ja01164a528
Return to citation in text: [1] [2] -
Hafner, K.; Moritz, K.-L. Justus Liebigs Ann. Chem. 1962, 656, 40–53. doi:10.1002/jlac.19626560108
Return to citation in text: [1] -
Kędziorek, M.; Mayer, P.; Mayr, H. Eur. J. Org. Chem. 2009, 1202–1206. doi:10.1002/ejoc.200801099
Return to citation in text: [1] -
Fraga, B. M. Nat. Prod. Rep. 2008, 25, 1180–1209. doi:10.1039/B806216C
Return to citation in text: [1] -
Faulkner, D. J. Nat. Prod. Rep. 1995, 12, 223–269. doi:10.1039/NP9951200223
Return to citation in text: [1] -
Seo, Y.; Rho, J.-R.; Geum, N.; Yoon, J. B.; Shin, J. J. Nat. Prod. 1996, 59, 985–986. doi:10.1021/np960485y
Return to citation in text: [1] -
Andersen, F. A. Int. J. Toxicol. 1999, 18 (Suppl. 3), 27–32. doi:10.1177/109158189901800304
Return to citation in text: [1] -
Barman, S.; Furukawa, H.; Blacque, O.; Venkatesan, K.; Yaghi, O. M.; Berke, H. Chem. Commun. 2010, 46, 7981–7983. doi:10.1039/c0cc02589e
Return to citation in text: [1] -
Wang, F.; Lai, Y.-H. Macromolecules 2003, 36, 536–538. doi:10.1021/ma025662i
Return to citation in text: [1] -
Zhang, X.-H.; Li, C.; Wang, W.-B.; Cheng, X.-X.; Wang, X.-S.; Zhang, B.-W. J. Mater. Chem. 2007, 17, 642–649. doi:10.1039/B613703B
Return to citation in text: [1] -
Ramadan, M.; Goeters, S.; Watzer, B.; Krause, E.; Lohmann, K.; Bauer, R.; Hempel, B.; Imming, P. J. Nat. Prod. 2006, 69, 1041–1045. doi:10.1021/np0601556
Return to citation in text: [1] -
Jakovlev, V.; Isaac, O.; Flaskamp, E. Planta Med. 1983, 49, 67–73. doi:10.1055/s-2007-969818
Return to citation in text: [1] -
Chen, C.-H.; Lee, O.; Yao, C.-N.; Chuang, M.-Y.; Chang, Y.-L.; Chang, M.-H.; Wen, Y.-F.; Yang, W.-H.; Ko, C.-H.; Chou, N.-T.; Lin, M.-W.; Lai, C.-P.; Sun, C.-Y.; Wang, L.-M.; Chen, Y.-C.; Hseu, T.-H.; Chang, C.-N.; Hsu, H.-C.; Lin, H.-C.; Chang, Y.-L.; Shih, Y.-C.; Chou, S.-H.; Hsu, Y.-L.; Tseng, H.-W.; Liu, C.-P.; Tu, C.-M.; Hu, T.-L.; Tsai, Y.-J.; Chen, T.-S.; Lin, C.-L.; Chiou, S.-J.; Liu, C.-C.; Hwang, C.-S. Bioorg. Med. Chem. Lett. 2010, 20, 6129–6132. doi:10.1016/j.bmcl.2010.08.025
Return to citation in text: [1] -
Safayhi, H.; Sabieraj, J.; Sailer, E.-R.; Ammon, H. P. T. Planta Med. 1994, 60, 410–413. doi:10.1055/s-2006-959520
Return to citation in text: [1] -
Fiori, J.; Teti, G.; Gotti, R.; Mazzotti, G.; Falconi, M. Toxicol. in Vitro 2011, 25, 64–72. doi:10.1016/j.tiv.2010.09.008
Return to citation in text: [1] -
Timmer, M. S. M.; Stocker, B. L.; Northcote, P. T.; Burkett, B. A. Tetrahedron Lett. 2009, 50, 7199–7204. doi:10.1016/j.tetlet.2009.10.043
Return to citation in text: [1] [2] -
Shoji, T.; Yokoyama, R.; Ito, S.; Watanabe, M.; Toyota, K.; Yasunami, M.; Morita, N. Tetrahedron Lett. 2007, 48, 1099–1103. doi:10.1016/j.tetlet.2006.12.083
Return to citation in text: [1] -
Shoji, T.; Okada, K.; Ito, S.; Toyota, K.; Morita, N. Tetrahedron Lett. 2010, 51, 5127–5130. doi:10.1016/j.tetlet.2010.07.090
Return to citation in text: [1] -
Kurotobi, K.; Tabata, H.; Miyauchi, M.; Murafuji, T.; Sugihara, Y. Synthesis 2002, 1013–1016. doi:10.1055/s-2002-31947
Return to citation in text: [1] -
Wakabayashi, S.; Kato, Y.; Mochizuki, K.; Suzuki, R.; Matsumoto, M.; Sugihara, Y.; Shimizu, M. J. Org. Chem. 2007, 72, 744–749. doi:10.1021/jo061684h
Return to citation in text: [1] -
Wakabayashi, S.; Uriu, R.; Asakura, T.; Akamatsu, C.; Sugihara, Y. Heterocycles 2008, 75, 383–390. doi:10.3987/COM-07-11229
Return to citation in text: [1] -
Ito, S.; Kubo, T.; Morita, N.; Matsui, Y.; Watanabe, T.; Ohta, A.; Fujimori, K.; Murafuji, T.; Sugihara, Y.; Tajiri, A. Tetrahedron Lett. 2004, 45, 2891–2894. doi:10.1016/j.tetlet.2004.02.059
Return to citation in text: [1] -
Higashi, J.; Shoji, T.; Ito, S.; Toyota, K.; Yasunami, M.; Morita, N. Eur. J. Org. Chem. 2008, 5823–5831. doi:10.1002/ejoc.200800733
Return to citation in text: [1] -
Merkul, E.; Oeser, T.; Müller, T. J. J. Chem.–Eur. J. 2009, 15, 5006–5011. doi:10.1002/chem.200900119
Return to citation in text: [1] [2] [3] -
Hafner, K.; Bangert, K.-F. Justus Liebigs Ann. Chem. 1961, 650, 98–115. doi:10.1002/jlac.19616500109
Return to citation in text: [1] -
Treibs, W.; Orttmann, H. Naturwissenschaften 1958, 45, 85–86. doi:10.1007/BF00632795
Return to citation in text: [1] -
Ito, S.; Okujima, T.; Kikuchi, S.; Shoji, T.; Morita, N.; Asao, T.; Ikoma, T.; Tero-Kubota, S.; Kawakami, J.; Tajiri, A. J. Org. Chem. 2008, 73, 2256–2263. doi:10.1021/jo702309b
Return to citation in text: [1] -
Reid, D. H.; Stafford, W. H.; Stafford, W. L. J. Chem. Soc. 1958, 1118–1127. doi:10.1039/JR9580001118
Return to citation in text: [1] -
Treibs, W. Chem. Ber. 1959, 92, 2152–2163. doi:10.1002/cber.19590920930
Return to citation in text: [1] -
Merkul, E.; Dohe, J.; Gers, C.; Rominger, F.; Müller, T. J. J. Angew. Chem., Int. Ed. 2011, 50, 2966–2969. doi:10.1002/anie.201007194
Return to citation in text: [1] -
Boersch, C.; Merkul, E.; Müller, T. J. J. Angew. Chemie, Int. Ed. 2011, in press.
Return to citation in text: [1] -
Willy, B.; Müller, T. J. J. Org. Lett. 2011, 13, 2082–2085. doi:10.1021/ol2004947
Return to citation in text: [1] -
Willy, B.; Müller, T. J. J. Eur. J. Org. Chem. 2008, 4157–4168. doi:10.1002/ejoc.200800444
Return to citation in text: [1] -
Wang, D.-L.; Deng, J.-J.; Xu, J.; Imafuku, K. Heterocycles 2007, 71, 2237–2242. doi:10.3987/COM-07-11101
Return to citation in text: [1]
| 39. | Timmer, M. S. M.; Stocker, B. L.; Northcote, P. T.; Burkett, B. A. Tetrahedron Lett. 2009, 50, 7199–7204. doi:10.1016/j.tetlet.2009.10.043 |
| 49. | Treibs, W.; Orttmann, H. Naturwissenschaften 1958, 45, 85–86. doi:10.1007/BF00632795 |
| 50. | Ito, S.; Okujima, T.; Kikuchi, S.; Shoji, T.; Morita, N.; Asao, T.; Ikoma, T.; Tero-Kubota, S.; Kawakami, J.; Tajiri, A. J. Org. Chem. 2008, 73, 2256–2263. doi:10.1021/jo702309b |
| 51. | Reid, D. H.; Stafford, W. H.; Stafford, W. L. J. Chem. Soc. 1958, 1118–1127. doi:10.1039/JR9580001118 |
| 1. | Müller, T. J. J.; D’Souza, D. M. Pure Appl. Chem. 2008, 80, 609–620. doi:10.1351/pac200880030609 |
| 2. | Müller, T. J. J. In Functional Organic Materials. Synthesis, Strategies, and Applications; Müller, T. J. J.; Bunz, U. H. F., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp 179–223. |
| 3. | Burke, M. D.; Schreiber, S. L. Angew. Chem., Int. Ed. 2004, 43, 46–58. doi:10.1002/anie.200300626 |
| 12. | Müller, T. J. J. Palladium-Copper Catalyzed Alkyne Activation as an Entry to Multicomponent Syntheses of Heterocycles. In Synthesis of Heterocycles via Multicomponent Reactions II; Orru, R. V. A.; Ruijter, E., Eds.; Topics in Heterocyclic Chemistry, Vol. 25; Springer: Berlin/Heidelberg, Germany, 2010; pp 25–94. doi:10.1007/7081_2010_43 |
| 13. | Willy, B.; Müller, T. J. J. Curr. Org. Chem. 2009, 13, 1777–1790. doi:10.2174/138527209789630479 |
| 14. | Willy, B.; Müller, T. J. J. ARKIVOC 2008, 2008 (i), 195–208. |
| 15. | Müller, T. J. J. Multi-component syntheses of heterocycles by virtue of palladium catalyzed generation of alkynones and chalcones. In Targets in Heterocyclic Systems; Attanasi, O.; Spinelli, D., Eds.; Italian Society of Chemistry: Rome, Italy, 2006; Vol. 10, pp 54–65. |
| 24. | Anderson, A. G., Jr.; Nelson, J. A. J. Am. Chem. Soc. 1950, 72, 3824–3825. doi:10.1021/ja01164a528 |
| 11. | D’Souza, D. M.; Müller, T. J. J. Chem. Soc. Rev. 2007, 36, 1095–1108. doi:10.1039/B608235C |
| 27. | Fraga, B. M. Nat. Prod. Rep. 2008, 25, 1180–1209. doi:10.1039/B806216C |
| 28. | Faulkner, D. J. Nat. Prod. Rep. 1995, 12, 223–269. doi:10.1039/NP9951200223 |
| 9. | Tietze, L. F. Chem. Rev. 1996, 96, 115–136. doi:10.1021/cr950027e |
| 10. | Coquerel, Y.; Boddaert, T.; Presset, M.; Mailhol, D.; Rodriguez, J. Multiple Bond-Forming Transformations: The Key Concept toward Eco-Compatible Synthetic Organic Chemistry. In Ideas in Chemistry and Molecular Sciences: Advances in Synthetic Chemistry; Pignataro, B., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp 187–202. doi:10.1002/9783527630554.ch9 |
| 23. | Hafner, K.; Bernhard, C.; Müller, R. Justus Liebigs Ann. Chem. 1961, 650, 35–41. doi:10.1002/jlac.19616500104 |
| 24. | Anderson, A. G., Jr.; Nelson, J. A. J. Am. Chem. Soc. 1950, 72, 3824–3825. doi:10.1021/ja01164a528 |
| 25. | Hafner, K.; Moritz, K.-L. Justus Liebigs Ann. Chem. 1962, 656, 40–53. doi:10.1002/jlac.19626560108 |
| 26. | Kędziorek, M.; Mayer, P.; Mayr, H. Eur. J. Org. Chem. 2009, 1202–1206. doi:10.1002/ejoc.200801099 |
| 55. | Willy, B.; Müller, T. J. J. Org. Lett. 2011, 13, 2082–2085. doi:10.1021/ol2004947 |
| 56. | Willy, B.; Müller, T. J. J. Eur. J. Org. Chem. 2008, 4157–4168. doi:10.1002/ejoc.200800444 |
| 4. | Sunderhaus, J. D.; Martin, S. F. Chem.–Eur. J. 2009, 15, 1300–1308. doi:10.1002/chem.200802140 |
| 5. | Isambert, N.; Lavilla, R. Chem.–Eur. J. 2008, 14, 8444–8454. doi:10.1002/chem.200800473 |
| 6. | Orru, R. V. A.; de Greef, M. Synthesis 2003, 1471–1499. doi:10.1055/s-2003-40507 |
| 7. | Touré, B. B.; Hall, D. G. Chem. Rev. 2009, 109, 4439–4486. doi:10.1021/cr800296p |
| 8. | Bonne, D.; Coquerel, Y.; Constantieux, T.; Rodriguez, J. Tetrahedron: Asymmetry 2010, 21, 1085–1109. doi:10.1016/j.tetasy.2010.04.045 |
| 23. | Hafner, K.; Bernhard, C.; Müller, R. Justus Liebigs Ann. Chem. 1961, 650, 35–41. doi:10.1002/jlac.19616500104 |
| 57. | Wang, D.-L.; Deng, J.-J.; Xu, J.; Imafuku, K. Heterocycles 2007, 71, 2237–2242. doi:10.3987/COM-07-11101 |
| 19. |
Zeller, K.-P. Houben-Weyl; 1985; Vol. 5/2c, pp 127–133.
and references cited therein. |
| 19. |
Zeller, K.-P. Houben-Weyl; 1985; Vol. 5/2c, pp 127–133.
and references cited therein. |
| 47. | Merkul, E.; Oeser, T.; Müller, T. J. J. Chem.–Eur. J. 2009, 15, 5006–5011. doi:10.1002/chem.200900119 |
| 21. | Pfau, A. S.; Plattner, P. A. Helv. Chim. Acta 1936, 19, 858–879. doi:10.1002/hlca.193601901117 |
| 22. | Plattner, P. A.; Pfau, A. S. Helv. Chim. Acta 1937, 20, 224–232. doi:10.1002/hlca.19370200133 |
| 54. | Boersch, C.; Merkul, E.; Müller, T. J. J. Angew. Chemie, Int. Ed. 2011, in press. |
| 16. | Hansen, H. J. Chimia 1996, 50, 489–496. |
| 17. | Hansen, H. J. Chimia 1997, 51, 147–159. |
| 1. | Müller, T. J. J.; D’Souza, D. M. Pure Appl. Chem. 2008, 80, 609–620. doi:10.1351/pac200880030609 |
| 2. | Müller, T. J. J. In Functional Organic Materials. Synthesis, Strategies, and Applications; Müller, T. J. J.; Bunz, U. H. F., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp 179–223. |
| 20. | Anderson, A. G., Jr.; Steckler, B. M. J. Am. Chem. Soc. 1959, 81, 4941–4946. doi:10.1021/ja01527a046 |
| 53. | Merkul, E.; Dohe, J.; Gers, C.; Rominger, F.; Müller, T. J. J. Angew. Chem., Int. Ed. 2011, 50, 2966–2969. doi:10.1002/anie.201007194 |
| 31. | Barman, S.; Furukawa, H.; Blacque, O.; Venkatesan, K.; Yaghi, O. M.; Berke, H. Chem. Commun. 2010, 46, 7981–7983. doi:10.1039/c0cc02589e |
| 32. | Wang, F.; Lai, Y.-H. Macromolecules 2003, 36, 536–538. doi:10.1021/ma025662i |
| 33. | Zhang, X.-H.; Li, C.; Wang, W.-B.; Cheng, X.-X.; Wang, X.-S.; Zhang, B.-W. J. Mater. Chem. 2007, 17, 642–649. doi:10.1039/B613703B |
| 29. | Seo, Y.; Rho, J.-R.; Geum, N.; Yoon, J. B.; Shin, J. J. Nat. Prod. 1996, 59, 985–986. doi:10.1021/np960485y |
| 30. | Andersen, F. A. Int. J. Toxicol. 1999, 18 (Suppl. 3), 27–32. doi:10.1177/109158189901800304 |
| 48. | Hafner, K.; Bangert, K.-F. Justus Liebigs Ann. Chem. 1961, 650, 98–115. doi:10.1002/jlac.19616500109 |
| 47. | Merkul, E.; Oeser, T.; Müller, T. J. J. Chem.–Eur. J. 2009, 15, 5006–5011. doi:10.1002/chem.200900119 |
| 45. | Ito, S.; Kubo, T.; Morita, N.; Matsui, Y.; Watanabe, T.; Ohta, A.; Fujimori, K.; Murafuji, T.; Sugihara, Y.; Tajiri, A. Tetrahedron Lett. 2004, 45, 2891–2894. doi:10.1016/j.tetlet.2004.02.059 |
| 46. | Higashi, J.; Shoji, T.; Ito, S.; Toyota, K.; Yasunami, M.; Morita, N. Eur. J. Org. Chem. 2008, 5823–5831. doi:10.1002/ejoc.200800733 |
| 47. | Merkul, E.; Oeser, T.; Müller, T. J. J. Chem.–Eur. J. 2009, 15, 5006–5011. doi:10.1002/chem.200900119 |
| 40. | Shoji, T.; Yokoyama, R.; Ito, S.; Watanabe, M.; Toyota, K.; Yasunami, M.; Morita, N. Tetrahedron Lett. 2007, 48, 1099–1103. doi:10.1016/j.tetlet.2006.12.083 |
| 41. | Shoji, T.; Okada, K.; Ito, S.; Toyota, K.; Morita, N. Tetrahedron Lett. 2010, 51, 5127–5130. doi:10.1016/j.tetlet.2010.07.090 |
| 42. | Kurotobi, K.; Tabata, H.; Miyauchi, M.; Murafuji, T.; Sugihara, Y. Synthesis 2002, 1013–1016. doi:10.1055/s-2002-31947 |
| 43. | Wakabayashi, S.; Kato, Y.; Mochizuki, K.; Suzuki, R.; Matsumoto, M.; Sugihara, Y.; Shimizu, M. J. Org. Chem. 2007, 72, 744–749. doi:10.1021/jo061684h |
| 44. | Wakabayashi, S.; Uriu, R.; Asakura, T.; Akamatsu, C.; Sugihara, Y. Heterocycles 2008, 75, 383–390. doi:10.3987/COM-07-11229 |
| 34. | Ramadan, M.; Goeters, S.; Watzer, B.; Krause, E.; Lohmann, K.; Bauer, R.; Hempel, B.; Imming, P. J. Nat. Prod. 2006, 69, 1041–1045. doi:10.1021/np0601556 |
| 35. | Jakovlev, V.; Isaac, O.; Flaskamp, E. Planta Med. 1983, 49, 67–73. doi:10.1055/s-2007-969818 |
| 36. | Chen, C.-H.; Lee, O.; Yao, C.-N.; Chuang, M.-Y.; Chang, Y.-L.; Chang, M.-H.; Wen, Y.-F.; Yang, W.-H.; Ko, C.-H.; Chou, N.-T.; Lin, M.-W.; Lai, C.-P.; Sun, C.-Y.; Wang, L.-M.; Chen, Y.-C.; Hseu, T.-H.; Chang, C.-N.; Hsu, H.-C.; Lin, H.-C.; Chang, Y.-L.; Shih, Y.-C.; Chou, S.-H.; Hsu, Y.-L.; Tseng, H.-W.; Liu, C.-P.; Tu, C.-M.; Hu, T.-L.; Tsai, Y.-J.; Chen, T.-S.; Lin, C.-L.; Chiou, S.-J.; Liu, C.-C.; Hwang, C.-S. Bioorg. Med. Chem. Lett. 2010, 20, 6129–6132. doi:10.1016/j.bmcl.2010.08.025 |
| 37. | Safayhi, H.; Sabieraj, J.; Sailer, E.-R.; Ammon, H. P. T. Planta Med. 1994, 60, 410–413. doi:10.1055/s-2006-959520 |
| 38. | Fiori, J.; Teti, G.; Gotti, R.; Mazzotti, G.; Falconi, M. Toxicol. in Vitro 2011, 25, 64–72. doi:10.1016/j.tiv.2010.09.008 |
| 39. | Timmer, M. S. M.; Stocker, B. L.; Northcote, P. T.; Burkett, B. A. Tetrahedron Lett. 2009, 50, 7199–7204. doi:10.1016/j.tetlet.2009.10.043 |
© 2011 Gers et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)