Abstract
The interaction of β- and γ-cyclodextrins (β-CD and γ-CD, respectively) with polyacrylamide modified with pyrenyl (Py) residues (pAAmPy) was investigated in a mixed solvent of water and dimethyl sulfoxide (DMSO) by steady-state fluorescence. In the absence of CD, the fluorescence spectra indicated that the formation of Py dimers became less favorable with increasing volume fraction of DMSO (xDMSO). The fluorescence spectra at varying xDMSO and CD concentrations indicated that β-CD and γ-CD included monomeric and dimeric Py residues, respectively. Using the fluorescence spectra, equilibrium constants of the formation of Py dimers and the complexation of β-CD and γ-CD with Py residues were roughly estimated based on simplified equilibrium schemes.

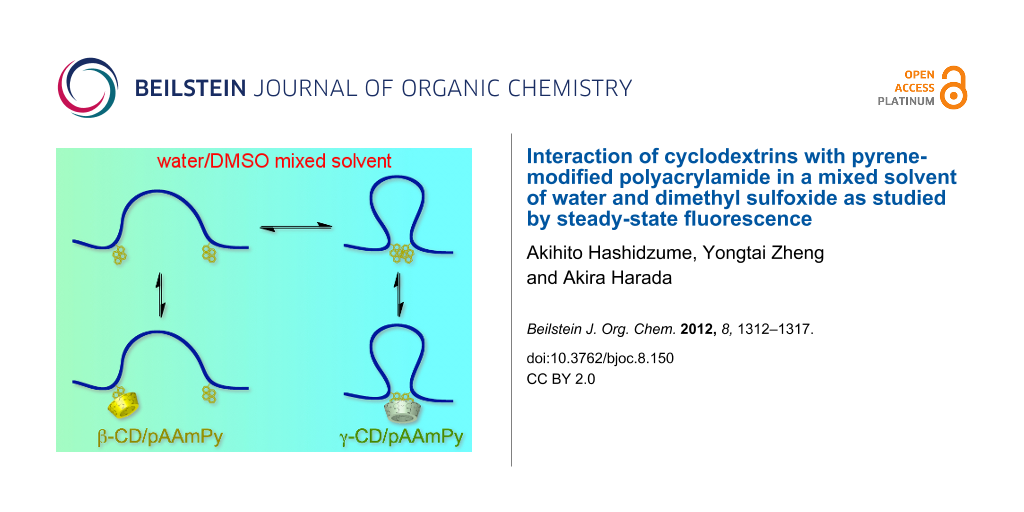
Graphical Abstract
Introduction
Cyclodextrins (CDs) are cyclic oligomers composed of glucopyranose units linked through α-1,4-glycoside bonding. They bear a tapered structure with a narrower rim of primary hydroxy groups and a wider rim of secondary hydroxy groups. CDs of 6, 7, and 8 glucopyranose units are called α-CD, β-CD, and γ-CD, respectively. CDs have a hydrophilic exterior and a rather hydrophobic cavity, and thus, recognize guest compounds of a size and shape matching their cavity, to form inclusion complexes [1-5]. Since CDs are nontoxic, they have been utilized in a variety of fields, including food additives, cosmetics, and personal care items [6-12]. In the past decade, the formation of inclusion complexes of CDs with guest residues attached on water-soluble polymers has attracted increasing interest from a number of research groups because these systems are applicable to stimuli-responsive systems [13-18].
We have been working on the interaction of CDs with water-soluble polymers bearing various guest residues, including linear, branched, and cyclic aliphatics, as well as aromatics [19-21], and realized stimuli-responsive hydrogels [22-27] and macroscopic assemblies based on molecular recognition [28-31]. Aromatic residues absorb light to become excited, and subsequently they can transfer energy and electrons. The interaction of CDs with water-soluble polymers carrying aromatic residues may allow one to construct functional systems that convert photo energy based on molecular recognition. Among aromatic compounds, pyrene is the most examined as a fluorescence probe or label because it shows a relatively high fluorescence quantum yield and a relatively long fluorescence lifetime in both monomer and excimer states [32,33]. Since pyrene is very hydrophobic, it may tend to form aggregates, e.g., dimers, in aqueous solutions. It is also known that pyrene forms inclusion complexes with β-CD and γ-CD in different manners; β-CD includes monomeric pyrene whereas γ-CD includes dimeric pyrene [34-36]. Recently, we have demonstrated this selectivity switching on macroscopic molecular recognition for polyacrylamide-based gels carrying pyrenyl (Py) and CD residues, by changing the composition of a mixed solvent of water and dimethyl sulfoxide (DMSO) [37]. In the present study, the interaction of β-CD and γ-CD with Py-modified polyacrylamide (pAAmPy, Scheme 1) was investigated in the water/DMSO mixed solvent of varying composition by steady-state fluorescence to elucidate the mechanism of the selectivity switching.
Results
Figure 1a demonstrates the steady-state fluorescence spectra measured for 0.04 g L−1 pAAmPy (5 μM in Py residue) at varying volume fractions of DMSO (xDMSO) in the water/DMSO mixed solvent in the absence of CD. At xDMSO = 0 (i.e., in water), the spectrum exhibits not only emission bands ascribable to monomeric Py in the region of 370–430 nm, but also a broad band assignable to a Py excimer around 480 nm, indicating that Py residues tend to form dimers because of the hydrophobicity. It is likely that Py residues associate intramolecularly under the dilute conditions (0.04 g L−1) in this study. These spectra indicate that the intensity of excimer fluorescence decreases whereas that of monomer fluorescence increases with increasing xDMSO. This observation indicates that the formation of Py dimer becomes less favorable, because the Py residue becomes more solvophilic with xDMSO. Using the spectra, the ratios (I480/I376) of the intensities at 480 and 376 nm, which are predominantly due to the Py excimer and monomer, respectively, were calculated and plotted in Figure 1b against xDMSO. I480/I376 decreases monotonously from 0.125 to 0.025 with increasing xDMSO from 0 to 1.
Figure 1: Steady-state fluorescence spectra for 0.04 g L−1 pAAmPy at varying xDMSO from 0 to 1 with excitation at 335 nm (a) and I480/I376 as a function of xDMSO (b).
Figure 1: Steady-state fluorescence spectra for 0.04 g L−1 pAAmPy at varying xDMSO from 0 to 1 with excitatio...
The interaction of β-CD and γ-CD with pAAmPy was also investigated at varying xDMSO by steady-state fluorescence. Figure 2 exhibits fluorescence spectra for the β-CD/pAAmPy system at xDMSO = 0.1 and for the γ-CD/pAAmPy system at xDMSO = 0 as typical examples, showing remarkable tendencies. In the spectra of the β-CD/pAAmPy system at xDMSO = 0.1, the intensity of Py excimer fluorescence decreases whereas that of Py monomer fluorescence increases with the increasing concentration of CD ([CD]0), indicating that β-CD forms inclusion complexes with monomeric Py residues, and dimeric Py residues dissociate to the monomers. In the spectra of the γ-CD/pAAmPy system at xDMSO = 0, on the other hand, the intensity of the excimer fluorescence increases whereas that of the monomer fluorescence decreases with increasing [CD]0, indicating that γ-CD forms inclusion complexes with dimeric Py residues, and monomeric Py residues further associate to form the dimers. Using the steady-state fluorescence spectra, I480/I376 values were calculated. Figure 3 compares I480/I376 as a function of [CD]0 for the β-CD/pAAmPy system at xDMSO = 0.1–0.6 and for the γ-CD/pAAmPy system at xDMSO = 0–0.2. At other xDMSO, I480/I376 was practically independent of [CD]0, indicative of no significant interaction of β-CD or γ-CD with pAAmPy. For the β-CD/pAAmPy system (Figure 3a), I480/I376 decreases with increasing [CD]0 at xDMSO = 0.1–0.6. For the γ-CD/pAAmPy system (Figure 3b), on the other hand, I480/I376 increases with [CD]0 at xDMSO = 0–0.2.
Figure 2: Steady-state fluorescence spectra for 0.04 g L−1 pAAmPy with excitation at 335 nm in the presence of varying concentrations of β-CD at xDMSO = 0.1 (a) and of γ-CD at xDMSO = 0 (b).
Figure 2: Steady-state fluorescence spectra for 0.04 g L−1 pAAmPy with excitation at 335 nm in the presence o...
Figure 3: I480/I376 as a function of [CD]0 for β-CD/pAAmPy (a) and γ-CD/pAAmPy (b) at different xDMSO.
Figure 3: I480/I376 as a function of [CD]0 for β-CD/pAAmPy (a) and γ-CD/pAAmPy (b) at different xDMSO.
Discussion
Detailed study of the equilibria of the inclusion complex formation of CDs with Py-modified water-soluble polymers, including the formation of the dynamic excimer, requires not only steady-state fluorescence measurements but also time-resolved fluorescence measurements [38-43]. In this study, however, equilibrium constants are roughly estimated by analyzing the steady-state fluorescence data, assuming that dynamic excimer formation is negligible. In the absence of CD, I480/I376 for pAAmPy decreases from 0.125 to 0.025 with increasing xDMSO from 0 to 1. It should be noted here that the fluorescence of the Py monomer is dominant compared to that of the Py excimer even at xDMSO = 0 (i.e., in water), implying that there are a significant fraction of Py residues (Py°) that cannot form Py dimers (Py2). Since the steady-state fluorescence measurements were performed under dilute conditions in this study, most of the Py2 were formed intramolecularly. Thus, Py residues in pAAmPy carrying a Py residue may not form Py2. The fraction of Py° is defined as f. Scheme 2a indicates a simplified equilibrium of the formation of Py2 from two Py residues. On the basis of the derivation of equations in the Supporting Information File 1, the equilibrium constant for the Py2 formation (KPy) can be calculated as
where [Py]0 is the total concentration of Py residue and x as given in Equation 2.
Here, A1,376, A1,480, A2,376, and A2,480 are constants corresponding to the products of the molar extinction coefficient and the fluorescence quantum yield (subscripts 1 and 2 indicate monomeric and dimeric Py residues, respectively, and subscripts 376 and 480 indicate the wavelengths), and B376 and B480 are constants corresponding to the background. If it is assumed that all the Py residues are in the monomer state at xDMSO = 1 (i.e., in DMSO), f = 0.5, A2,480/A1,376 = 0.5, and B376 = B480 = 0, KPy can be calculated as can be seen in Figure 4a. This figure indicates that KPy decreases monotonously from 6.2 × 104 to 0 M−1 with increasing xDMSO from 0 to 1.
Scheme 2: Simplified equilibria of CDs/pAAmPy systems.
Scheme 2: Simplified equilibria of CDs/pAAmPy systems.
Figure 4: KPy, Kβ, and Kγ as a function of xDMSO.
Figure 4: KPy, Kβ, and Kγ as a function of xDMSO.
In the β-CD/pAAmPy system, β-CD forms inclusion complexes with both Py and Py° (Scheme 2b). On the basis of the derivation described in the Supporting Information File 1, the concentrations of all species can be calculated by using the equilibrium constant (Kβ) for the inclusion complex formation, and I480/I376 can be also obtained as given in Equation 3.
Here [Py], [Py°], [CD], [CD·Py], and [CD·Py°] denote the concentrations of Py, Py°, free CD, and the complexes of CD with Py and with Py°, respectively, and A'1,376 and A'1,480 are constants. In this study, A'1,480/A'1,376 is fixed at 0.025 (Supporting Information File 1). It is also likely that A2,376 = 0. When Kβ and A'1,376/A1,376 are chosen appropriately, the calculated I480/I376 values agree with the experimental data, as can be seen in Figure 3a. The Kβ values were plotted in Figure 4b against xDMSO. As xDMSO increases from 0.1 to 0.6, Kβ decreases from 4 × 102 to 8 × 101 M−1. This observation indicates that the formation of inclusion complexes becomes less favorable with increasing xDMSO.
In the γ-CD/pAAmPy system, γ-CD forms inclusion complexes with Py2, in which Py° is not involved. On the basis of the derivation described in the Supporting Information File 1, the concentrations of all species can be calculated by using the equilibrium constant (Kγ) for the inclusion complex formation, and I480/I376 can be also obtained as given in Equation 4.
Here [CD·Py2] denotes the concentration of the complex of CD with Py2, and A'2,480 is a constant. It is also likely that A2,376 = A'2,376 = 0. When Kγ and A'2,480/A1,376 are chosen appropriately, the I480/I376 values calculated agree with the experimental data, as can be seen in Figure 3b. The Kγ values were also plotted in Figure 4b against xDMSO. This figure indicates that Kγ is practically constant (ca. 1.5 × 103 M–1) independent of xDMSO in the region of 0 ≤ xDMSO ≤ 0.2.
It should be noted here that the values of KPy, Kβ, and Kγ were estimated rather qualitatively based on the simplified equilibria and a number of assumptions, but the Kβ and Kγ values are in good agreement with the values reported for pyrene (4.9 × 102 and 1.1 × 103 M−1 for β-CD and γ-CD, respectively) [44].
Experimental
1-Pyrenemethylamine hydrochloride was purchased from Sigma-Aldrich Co. Ltd. Acryloyl chloride was obtained from Tokyo Chemical Industry Co. Ltd. Triethylamine, acrylamide (AAm), ammonium peroxodisulfate (APS), acetone, methanol, DMSO (spectroscopic grade), NaHCO3, and NaOH were purchased from Nacalai Tesque Inc. N,N-Dimethylformamide (DMF) and dichloromethane (DCM) were purified by utilizing a glass contour solvent dispensing system. Water was purified by a Millipore Milli-Q system. β-CD and γ-CD were purchased from Junsei Chemical Co. Ltd. and recrystallized twice from water before use. N-1-Pyrenylmethylacrylamide (APy) was prepared from 1-pyrenemethylamine hydrochloride and acryloyl chloride according to the procedure reported previously [37]. Other reagents were reagent grade and used without further purification.
The polymer (pAAmPy) was prepared by radical copolymerization of AAm and APy using APS as the initiator. A predetermined amount of AAm and APy were dissolved in DMF. After purging with dry argon for 30 min, APS (3 mg, 13 μmol) was added to the monomer solution. The reaction mixture was placed into a cuvette equipped with a stirrer and sealed. The cuvette was warmed with an oil bath thermostated at 60 °C overnight. The reaction mixture was poured into an excess of methanol to give a precipitate. The polymer obtained was recovered by filtration and dried under vacuum. The molecular weight of the polymer was estimated to be 4 × 103 by size exclusion chromatography (SEC), and the Py content was determined to be ca. 1 mol % by 1H NMR.
Steady-state fluorescence spectra were obtained on a HITACHI F-2500 spectrophotometer with excitation at 335 nm by using a 1 cm quartz cuvette. The slit widths for both excitation and emission sides were kept at 2.5 nm during measurement. SEC analysis was carried out at 40 °C on a TOSOH CCP & 8020 system equipped with two TOSOH TSKgel α-M columns connected in series, using formamide as the eluent at a flow rate of 0.3 mL min−1. TOSOH UV-8020 and TOSOH RI-8021 detectors were used. The molecular weights were calibrated by polystyrene sulfonate sodium-salt samples (American Polymer Standards). 1H NMR spectra were measured on a JEOL JNM-ECA500 spectrometer by using a mixed solvent of DMSO-d6 and D2O (1/1, v/v) as a solvent, and chemical shifts were referenced to the solvent value (i.e., 2.49 ppm for DMSO).
Supporting Information
| Supporting Information File 1: Equilibria for the CDs/pAAmPy systems. | ||
| Format: PDF | Size: 160.3 KB | Download |
References
-
Bender, M. L.; Komiyama, M. Cyclodextrin Chemistry; Springer: Berlin, Germany, 1978.
Return to citation in text: [1] -
Szejtli, J. Cyclodextrins and Their Inclusion Complexes; Akadémiai Kiadó: Budapest, Hungury, 1982.
Return to citation in text: [1] -
Szejtli, J.; Osa, T., Eds. Cyclodextrins; Pergamon: Oxford, U.K., 1996.
Return to citation in text: [1] -
Harada, A. In Large Ring Molecules; Semlyen, J. A., Ed.; Wiley & Sons: Chichester, U.K., 1996; pp 407–432.
Return to citation in text: [1] -
Dodziuk, H., Ed. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Wiley-VCH: Weinheim, Germany, 2006.
Return to citation in text: [1] -
Uekama, K.; Hirayama, F.; Irie, T. Chem. Rev. 1998, 98, 2045–2076. doi:10.1021/cr970025p
Return to citation in text: [1] -
Uekama, K.; Hirayama, F.; Arima, H. Pharmaceutical Applications of Cyclodextrins and Their Derivatives. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp 381–422. doi:10.1002/3527608982.ch14
Return to citation in text: [1] -
Hashimoto, H. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp 452–459.
Return to citation in text: [1] -
Szejtli, J.; Szente, L. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. doi:10.1016/j.ejpb.2005.05.006
Return to citation in text: [1] -
Brewster, M. E.; Loftsson, T. Adv. Drug Delivery Rev. 2007, 59, 645–666. doi:10.1016/j.addr.2007.05.012
Return to citation in text: [1] -
Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J. C.; Rial-Otero, R.; Simal-Gándara, J. Food Hydrocolloids 2009, 23, 1631–1640. doi:10.1016/j.foodhyd.2009.01.001
Return to citation in text: [1] -
Loftsson, T.; Brewster, M. E. J. Pharm. Pharmacol. 2010, 62, 1607–1621. doi:10.1111/j.2042-7158.2010.01030.x
Return to citation in text: [1] -
Wenz, G.; Han, B.-H.; Müller, A. Chem. Rev. 2006, 106, 782–817. doi:10.1021/cr970027+
Return to citation in text: [1] -
Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Chem. Rev. 2009, 109, 5974–6023. doi:10.1021/cr9000622
Return to citation in text: [1] -
Zhou, J.; Ritter, H. Polym. Chem. 2010, 1, 1552–1559. doi:10.1039/c0py00219d
Return to citation in text: [1] -
Yuen, F.; Tam, K. C. Soft Matter 2010, 6, 4613–4630. doi:10.1039/c0sm00043d
Return to citation in text: [1] -
Chen, G.; Jiang, M. Chem. Soc. Rev. 2011, 40, 2254–2266. doi:10.1039/c0cs00153h
Return to citation in text: [1] -
Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric Materials; John Wiley & Sons, Inc., 2012; pp 1–31.
Return to citation in text: [1] -
Hashidzume, A.; Tomatsu, I.; Harada, A. Polymer 2006, 47, 6011–6027. doi:10.1016/j.polymer.2006.06.021
Return to citation in text: [1] -
Harada, A.; Hashidzume, A. Aust. J. Chem. 2010, 63, 599–610. doi:10.1071/CH09609
Return to citation in text: [1] -
Hashidzume, A.; Harada, A. Polym. Chem. 2011, 2, 2146–2154. doi:10.1039/c1py00162k
Return to citation in text: [1] -
Tomatsu, I.; Hashidzume, A.; Harada, A. Macromolecules 2005, 38, 5223–5227. doi:10.1021/ma050670v
Return to citation in text: [1] -
Tomatsu, I.; Hashidzume, A.; Harada, A. Macromol. Rapid Commun. 2006, 27, 238–241. doi:10.1002/marc.200500793
Return to citation in text: [1] -
Tomatsu, I.; Hashidzume, A.; Harada, A. J. Am. Chem. Soc. 2006, 128, 2226–2227. doi:10.1021/ja058345a
Return to citation in text: [1] -
Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Angew. Chem., Int. Ed. 2010, 49, 7461–7464. doi:10.1002/anie.201003567
Return to citation in text: [1] -
Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Eur. J. Org. Chem. 2011, 2801–2806. doi:10.1002/ejoc.201100077
Return to citation in text: [1] -
Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Nat. Commun. 2011, 2, No. 511. doi:10.1038/ncomms1521
Return to citation in text: [1] -
Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Nat. Chem. 2011, 3, 34–37. doi:10.1038/nchem.893
Return to citation in text: [1] -
Yamaguchi, H.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A. Macromolecules 2011, 44, 2395–2399. doi:10.1021/ma200398y
Return to citation in text: [1] -
Zheng, Y.; Hashidzume, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Langmuir 2011, 27, 13790–13795. doi:10.1021/la2034142
Return to citation in text: [1] -
Yamaguchi, H.; Kobayashi, Y.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A. Nat. Commun. 2012, 3, No. 603. doi:10.1038/ncomms1617
Return to citation in text: [1] -
Turro, N. J. Modern Molecular Photochemistry; University Science Books: Sausalito, CA, 1991.
Return to citation in text: [1] -
Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2002.
Return to citation in text: [1] -
Yorozu, T.; Hoshino, M.; Imamura, M. J. Phys. Chem. 1982, 86, 4426–4429. doi:10.1021/j100219a031
Return to citation in text: [1] -
Hamai, S. J. Phys. Chem. 1989, 93, 6527–6529. doi:10.1021/j100354a048
Return to citation in text: [1] -
Ueno, A. Supramol. Sci. 1996, 3, 31–36. doi:10.1016/0968-5677(96)00016-8
Return to citation in text: [1] -
Zheng, Y.; Hashidzume, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Nat. Commun. 2012, 3, No. 831. doi:10.1038/ncomms1841
Return to citation in text: [1] [2] -
Siu, H.; Duhamel, J. J. Phys. Chem. B 2008, 112, 15301–15312. doi:10.1021/jp801105q
Return to citation in text: [1] -
Siu, H.; Duhamel, J. J. Phys. Chem. B 2012, 116, 1226–1233. doi:10.1021/jp208168r
Return to citation in text: [1] -
Duhamel, J. Polymers 2012, 4, 211–239. doi:10.3390/polym4010211
Return to citation in text: [1] -
Duhamel, J. Langmuir 2012, 28, 6527–6538. doi:10.1021/la2047646
Return to citation in text: [1] -
de Melo, J. S.; Costa, T.; da G. Miguel, M.; Lindman, B.; Schillén, K. J. Phys. Chem. B 2003, 107, 12605–12621. doi:10.1021/jp0346054
Return to citation in text: [1] -
de Melo, J. S. S.; Costa, T.; Oliveira, N.; Schillén, K. Polym. Int. 2007, 56, 882–899. doi:10.1002/pi.2219
Return to citation in text: [1] -
Blyshak, L. A.; Warner, I. M.; Patonay, G. Anal. Chim. Acta 1990, 232, 239–243. doi:10.1016/S0003-2670(00)81238-6
Return to citation in text: [1]
| 1. | Bender, M. L.; Komiyama, M. Cyclodextrin Chemistry; Springer: Berlin, Germany, 1978. |
| 2. | Szejtli, J. Cyclodextrins and Their Inclusion Complexes; Akadémiai Kiadó: Budapest, Hungury, 1982. |
| 3. | Szejtli, J.; Osa, T., Eds. Cyclodextrins; Pergamon: Oxford, U.K., 1996. |
| 4. | Harada, A. In Large Ring Molecules; Semlyen, J. A., Ed.; Wiley & Sons: Chichester, U.K., 1996; pp 407–432. |
| 5. | Dodziuk, H., Ed. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Wiley-VCH: Weinheim, Germany, 2006. |
| 22. | Tomatsu, I.; Hashidzume, A.; Harada, A. Macromolecules 2005, 38, 5223–5227. doi:10.1021/ma050670v |
| 23. | Tomatsu, I.; Hashidzume, A.; Harada, A. Macromol. Rapid Commun. 2006, 27, 238–241. doi:10.1002/marc.200500793 |
| 24. | Tomatsu, I.; Hashidzume, A.; Harada, A. J. Am. Chem. Soc. 2006, 128, 2226–2227. doi:10.1021/ja058345a |
| 25. | Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Angew. Chem., Int. Ed. 2010, 49, 7461–7464. doi:10.1002/anie.201003567 |
| 26. | Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Eur. J. Org. Chem. 2011, 2801–2806. doi:10.1002/ejoc.201100077 |
| 27. | Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Nat. Commun. 2011, 2, No. 511. doi:10.1038/ncomms1521 |
| 19. | Hashidzume, A.; Tomatsu, I.; Harada, A. Polymer 2006, 47, 6011–6027. doi:10.1016/j.polymer.2006.06.021 |
| 20. | Harada, A.; Hashidzume, A. Aust. J. Chem. 2010, 63, 599–610. doi:10.1071/CH09609 |
| 21. | Hashidzume, A.; Harada, A. Polym. Chem. 2011, 2, 2146–2154. doi:10.1039/c1py00162k |
| 13. | Wenz, G.; Han, B.-H.; Müller, A. Chem. Rev. 2006, 106, 782–817. doi:10.1021/cr970027+ |
| 14. | Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Chem. Rev. 2009, 109, 5974–6023. doi:10.1021/cr9000622 |
| 15. | Zhou, J.; Ritter, H. Polym. Chem. 2010, 1, 1552–1559. doi:10.1039/c0py00219d |
| 16. | Yuen, F.; Tam, K. C. Soft Matter 2010, 6, 4613–4630. doi:10.1039/c0sm00043d |
| 17. | Chen, G.; Jiang, M. Chem. Soc. Rev. 2011, 40, 2254–2266. doi:10.1039/c0cs00153h |
| 18. | Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric Materials; John Wiley & Sons, Inc., 2012; pp 1–31. |
| 6. | Uekama, K.; Hirayama, F.; Irie, T. Chem. Rev. 1998, 98, 2045–2076. doi:10.1021/cr970025p |
| 7. | Uekama, K.; Hirayama, F.; Arima, H. Pharmaceutical Applications of Cyclodextrins and Their Derivatives. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp 381–422. doi:10.1002/3527608982.ch14 |
| 8. | Hashimoto, H. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp 452–459. |
| 9. | Szejtli, J.; Szente, L. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. doi:10.1016/j.ejpb.2005.05.006 |
| 10. | Brewster, M. E.; Loftsson, T. Adv. Drug Delivery Rev. 2007, 59, 645–666. doi:10.1016/j.addr.2007.05.012 |
| 11. | Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J. C.; Rial-Otero, R.; Simal-Gándara, J. Food Hydrocolloids 2009, 23, 1631–1640. doi:10.1016/j.foodhyd.2009.01.001 |
| 12. | Loftsson, T.; Brewster, M. E. J. Pharm. Pharmacol. 2010, 62, 1607–1621. doi:10.1111/j.2042-7158.2010.01030.x |
| 37. | Zheng, Y.; Hashidzume, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Nat. Commun. 2012, 3, No. 831. doi:10.1038/ncomms1841 |
| 44. | Blyshak, L. A.; Warner, I. M.; Patonay, G. Anal. Chim. Acta 1990, 232, 239–243. doi:10.1016/S0003-2670(00)81238-6 |
| 34. | Yorozu, T.; Hoshino, M.; Imamura, M. J. Phys. Chem. 1982, 86, 4426–4429. doi:10.1021/j100219a031 |
| 35. | Hamai, S. J. Phys. Chem. 1989, 93, 6527–6529. doi:10.1021/j100354a048 |
| 36. | Ueno, A. Supramol. Sci. 1996, 3, 31–36. doi:10.1016/0968-5677(96)00016-8 |
| 37. | Zheng, Y.; Hashidzume, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Nat. Commun. 2012, 3, No. 831. doi:10.1038/ncomms1841 |
| 32. | Turro, N. J. Modern Molecular Photochemistry; University Science Books: Sausalito, CA, 1991. |
| 33. | Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2002. |
| 28. | Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Nat. Chem. 2011, 3, 34–37. doi:10.1038/nchem.893 |
| 29. | Yamaguchi, H.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A. Macromolecules 2011, 44, 2395–2399. doi:10.1021/ma200398y |
| 30. | Zheng, Y.; Hashidzume, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Langmuir 2011, 27, 13790–13795. doi:10.1021/la2034142 |
| 31. | Yamaguchi, H.; Kobayashi, Y.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A. Nat. Commun. 2012, 3, No. 603. doi:10.1038/ncomms1617 |
| 38. | Siu, H.; Duhamel, J. J. Phys. Chem. B 2008, 112, 15301–15312. doi:10.1021/jp801105q |
| 39. | Siu, H.; Duhamel, J. J. Phys. Chem. B 2012, 116, 1226–1233. doi:10.1021/jp208168r |
| 40. | Duhamel, J. Polymers 2012, 4, 211–239. doi:10.3390/polym4010211 |
| 41. | Duhamel, J. Langmuir 2012, 28, 6527–6538. doi:10.1021/la2047646 |
| 42. | de Melo, J. S.; Costa, T.; da G. Miguel, M.; Lindman, B.; Schillén, K. J. Phys. Chem. B 2003, 107, 12605–12621. doi:10.1021/jp0346054 |
| 43. | de Melo, J. S. S.; Costa, T.; Oliveira, N.; Schillén, K. Polym. Int. 2007, 56, 882–899. doi:10.1002/pi.2219 |
© 2012 Hashidzume et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)