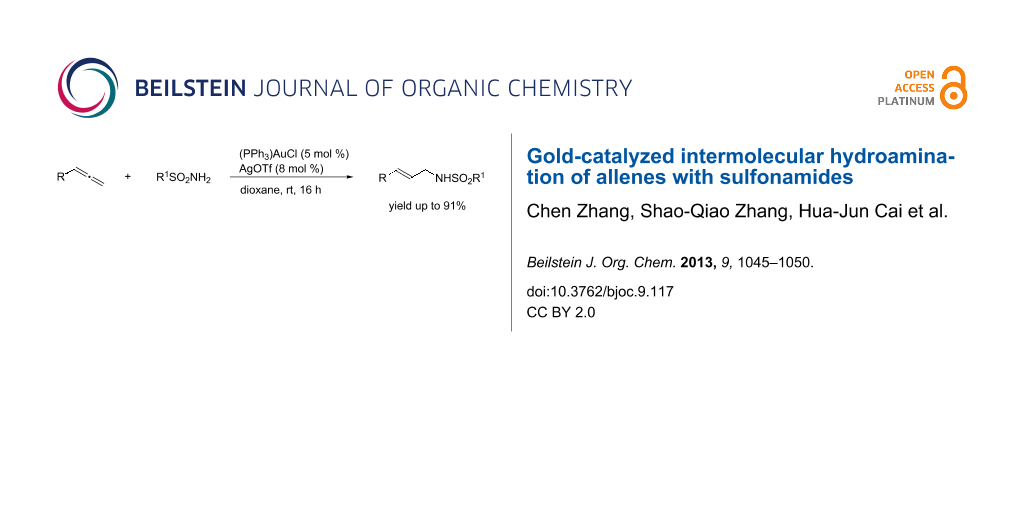
Abstract
A co-catalyst of (PPh3)AuCl/AgOTf for the intermolecular hydroamination of allenes with sulfonamides is shown. The reaction proceeded smoothly under mild conditions for differently substituted allenes giving N-allylic sulfonamides in good yields with high regioselectivity and E-selectivity.
Graphical Abstract
Introduction
Hydroamination of an N–H bond across a C–C unsaturated bond represents one of the most effective and atom-economical methods to prepare amine derivatives [1-5]. In the case of using allenes, this reaction can lead to allylamines, which are invaluable precursors for the synthesis of natural products and other potentially biologically relevant substances [6]. In the literature, a wide range of catalytic intramolecular hydroaminations of allenes are known, but only a small number of intermolecular hydroamination reactions are reported [7-15]. More recently, Au(I), Au(III), Pt(II) and Rh(I) have been used for the intermolecular hydroamination of allenes with secondary alkylamines, ammonia, or carboxamide [7,16-24]. Although some of these advances have been efficiently made in hydroamination, many require extreme and extended reaction conditions. Thus, development of these reactions is still needed. Recently, Yamamoto and co-workers reported the Pd(0)-catalyzed intermolecular hydroamination of allenes with sulfonamides [25]. In this paper, we wish to develop a gold(I)-complex-catalyzed addition of sulfonamides as the amine partner to allenes to synthesize N-allylic sulfonamides with high regio- and stereoselectivity.
Results and Discussion
As part of our ongoing studies on metal-catalyzed reactions, we have reported the hydroalkoxylation of allenes with alcohols and hydroamination of alkynes with sulfonamides in the presence of gold catalysts [26-28]. On the basis of these studies, in an initial experiment, 1-phenyl-1,2-propadiene (1a) (1.5 mmol) was treated with 4-methylbenzenesufonamide (2a) (0.5 mmol) in the presence of 2 mol % of (PPh3)AuCl and 8 mol % of AgOTf in dioxane at 70 °C efficiently to form linear adduct 3a in 43% yield (Table 1, entry 1). Different solvents were screened, and dioxane was found to be the most suitable one (Table 1, entries 2–4). Decreasing the amount of AgOTf resulted in lower yields (Table 1, entry 7). We were pleased to find that efficient hydroamination was realized at rt and led to a 91% yield of 3a with good regioselectivity and high E-selectivity (Table 1, entry 6). Other possible isomers could not be detected. As Ag catalysts, other salts were also screened, AgBF4 was ineffective. With AgSbF6 or AgNTf2, the reaction took place and gave adducts in 33% and 47% yield (Table 1, entries 8 and 9). The use of the gold alone gave a lower yield, and the reaction did not proceed in the absence of gold or through the use of TfOH (entries 11–14). Finally, we determined the optimal conditions as 5 mol % of (PPh3)AuCl and 8 mol % of AgOTf in dioxane at rt (Table 1, entry 6).
Table 1: Catalytic hydroamination of 1a and 2a.a
|
|
||||||
| Entry | [Au] (mol %) | [Ag] (mol %) | Solvent | Time (h) | Temp. (°C) | Yield (%)b |
|---|---|---|---|---|---|---|
| 1 | (PPh3)AuCl (2) | AgOTf (8) | dioxane | 4 | 70 | 43 |
| 2 | (PPh3)AuCl (2) | AgOTf (8) | THF | 6 | 70 | 28 |
| 3 | (PPh3)AuCl (2) | AgOTf (8) | toluene | 8 | 70 | 32 |
| 4 | (PPh3)AuCl (2) | AgOTf (8) | (CH2Cl)2 | 7 | 70 | 33 |
| 5 | (PPh3)AuCl (5) | AgOTf (8) | dioxane | 4 | 70 | 59 |
| 6 | (PPh3)AuCl (5) | AgOTf (8) | dioxane | 16 | rt | 91 |
| 7 | (PPh3)AuCl (5) | AgOTf (5) | dioxane | 16 | rt | 80 |
| 8 | (PPh3)AuCl (5) | AgSbF6 (8) | dioxane | 24 | rt | 33 |
| 9 | (PPh3)AuCl (5) | AgNTf2 (8) | dioxane | 24 | rt | 47 |
| 10 | Au(NHC)Cl (5) | AgOTf (8) | dioxane | 16 | rt | 46 |
| 11 | 0 | 0 | dioxane | 16 | rt | 0 |
| 12 | (PPh3)AuCl (5) | 0 | dioxane | 16 | rt | 26 |
| 13 | 0 | AgOTf (8) | dioxane | 16 | rt | 0 |
| 14 | 0 | TfOH (8) | dioxane | 16 | rt | 0 |
aAll reactions were performed with 0.8 mmol of 1a, 0.4 mmol of 2a, 0–5 mol % of (PPh3)AuCl, and 0–8 mol % of AgOTf. bIsolated yields.
To further assess the scope of this process, we first examined the hydroamination of 1a with several sulfonamides. Benzenesulfonamides containing p-Br or p-Cl groups on the benzene ring were tolerated for the reaction, obtaining the corresponding adducts 3d and 3e in 54 and 72% yields, respectively (Table 2, entries 4–5). Under the same reaction conditions, the hydroamination of aliphatic sulfonamides took place smoothly to afford the corresponding N-allylic sulfonamide 3f with 56% yield (Table 2, entry 6). We also used N-substituted sulfonamide 2g as the amine partner. Although drastic conditions are required, the addition occurred to provide linear adduct 3g in good yield (Table 2, entry 7). In all cases, the adduct was obtained with high selectivity.
Table 2: Hydroamination of 1a with sulfonamide 2.a
| Entry | Sulfonamide | 2 | Product | 3 | Yield (%)b |
|---|---|---|---|---|---|
| 1 | TsNH2 | 2a |
|
3a | 91 |
| 2 | PhSO2NH2 | 2b |
|
3b | 76 |
| 3 | o-Me-C6H4SO2NH2 | 2c |
|
3c | 60 |
| 4 | p-Br-C6H4SO2NH2 | 2d |
|
3d | 54 |
| 5 | p-Cl-C6H4SO2NH2 | 2e |
|
3e | 72 |
| 6 | MeSO2NH2 | 2f |
|
3f | 56 |
| 7c | n-BuNHTs | 2g |
|
3g | 79 |
aThe reactions were performed with 0.8 mmol of allene 1a, 0.4 mmol of 2, 5 mol % of (Ph3P)AuCl and 8 mol % of AgOTf in 2 mL of dioxane at rt for 16 h. bIsolated yield. cAt 100 °C for 8 h.
Various allenes were then examined, and aromatic rings of phenylallenes with either an electron-donating or an electron-withdrawing group gave good isolated yields of the corresponding adducts (Table 3, entries 1 and 2). Whereas hydroamination of the monosubstituted heteroaromatic allene 1d also lead to the conversion into the expected addition product 3j, hydroamination of the monoalkyl-substituted aliphatic allene 1e formed a 71:29 mixture of linear product (3ka) and branch product (3kb) under the same conditions (Table 3, entry 4). Furthermore, disubstituted allenes also worked well. Differentially 1,1-disubstituted allene 1f reacted with sulfonamide to afford trans-adducts 3l with high selectivity (Table 3, entry 5). Single crystals of the compound 3l suitable for X-ray crystallographic analysis were also obtained (Figure 1). This shows that 3l is the E isomer, the sulfonamide carbon link being trans to the phenyl group (Figure 1). As for differentially 1,3- disubstituted allene 1g, hydroamination took place with exclusive attack of sulfonamide at the more electron-rich allene terminus to afford the corresponding adduct 3m with 68% yield and with high E-selectivity (Table 3, entry 6). In addition, hydroamination of trisubstituted allene 1h took place to afford a different product (Table 3, entry 7). Single crystals of the compound 3n suitable for X-ray crystallographic analysis were also obtained (Figure 2). This showed that 3n is the E isomer, the sulfonamide being trans to the diphenylmethyl groups (Figure 2).
Table 3: Hydroamination of allenes 1 with 2a.a
| Entry | Allene | 1 | Product | 3 | Yield (%) |
|---|---|---|---|---|---|
| 1 |
|
1b |
|
3h | 82 |
| 2 |
|
1c |
|
3i | 72 |
| 3 |
|
1d |
|
3j | 60 |
| 4 |
|
1e |
|
3ka
3kb |
48 |
| 5 |
|
1f |
|
3l | 67 |
| 6 |
|
1g |
|
3m | 68 |
| 7 |
|
1h |
|
3n | 31 |
aThe reactions were performed with 0.8 mmol of allene 1, 0.4 mmol of 2a, 5 mol % of (Ph3P)AuCl and 8 mol % of AgOTf in 2 mL of dioxane at rt for 16 h. bIsolated yield. c3ka/3kb = 71:29.
The proposed mechanism of the gold-catalyzed hydroaamination of allenes is shown in Scheme 1 [2,7,29-36]. The gold cation coordinated with allene to form cationic Au(I)-allene complex A, and this leads to cationic gold(I) complex B. The sulfonamide attacks at the less-substituted terminus of intermediate B to form C. Protonolysis of the Au–C bond of B yields the allylic sulfonamide 3, regenerating the gold complex. On the other hand, in comparison with phenyl-substituted allenes, for alkyl-substituted allene 1e, a mixture of 3ka and 3kb was produced; although the details are unclear, perhaps due to electronic factors, the addition of sulfonamide also occurred at the more-hindered position of intermediate B to give 3ka and 3kb.
Scheme 1: Proposed mechanism for the hydroamination of allenes.
Scheme 1: Proposed mechanism for the hydroamination of allenes.
Conclusion
In conclusion, we have successfully employed (PPh3)AuCl/AgOTf catalyzed intermolecular hydroamination of allenes with sulfonamides to produce N-allylic sulfonamide. This reaction takes place under mild conditions with effective and high regio- and stereoselectivity. Monosubstituted, 1,1- and 1,3-disubstituted, and trisubstituted allenes were well tolerated in the reaction.
Experimental
General Information: Unless otherwise noted, materials were obtained from commercial suppliers and used without further purification. Allenes were prepared by procedures in the literature [37-39]. Thin-layer chromatography (TLC) was performed on glass plates coated with silica gel 60 F254 and visualized by UV light (254 nm). Column chromatography was performed with silica gel (mesh 300–400). Infrared (IR) spectra were obtained on a 370 FTIR spectrometer; absorptions are reported in cm−1. Mass spectra were obtained in the electron impact (EI) mode, and high-resolution mass spectra were measured on a high-resolution mass spectrometer (GCT Premier).
General Procedure: To a mixture of sulfonamide (0.4 mmol), PPh3AuCl (0.02 mmol), and AgOTf (0.032 mmol) in anhydrous 1,4-dioxane (2 mL) was added allene (0.8 mmol). The mixture was then sealed and stirred at room temperature until the starting sulfonamide was consumed as judged by TLC. The mixture was quenched with a saturated solution of NaHCO3 and then extracted with ethyl acetate (3 × 20 mL). The organic layer was washed with brine, dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (silica gel) to yield the product in an analytically pure form.
Supporting Information
| Supporting Information File 1: Analytical and spectroscopic data for compounds 3a–3j, 3ka, 3kb and 3l–3n. | ||
| Format: PDF | Size: 6.8 MB | Download |
References
-
Müller, T. E.; Hultzsch, K. C.; Yus, F.; Foubelo, M.; Tada, M. Chem. Rev. 2008, 108, 3795. doi:10.1021/cr0306788
Return to citation in text: [1] -
Widenhoefer, R. A.; Han, X. Eur. J. Org. Chem. 2006, 4555. doi:10.1002/ejoc.200600399
Return to citation in text: [1] [2] -
Pohlki, F.; Doye, S. Chem. Soc. Rev. 2003, 32, 104. doi:10.1039/b200386b
Return to citation in text: [1] -
Hong, S.; Marks, T. J. Acc. Chem. Res. 2004, 37, 673. doi:10.1021/ar040051r
Return to citation in text: [1] -
Hultzsch, K. C. Adv. Synth. Catal. 2005, 347, 367. doi:10.1002/adsc.200404261
Return to citation in text: [1] -
Johannsen, M.; Jørgensen, K. A. Chem. Rev. 1998, 98, 1689. doi:10.1021/cr970343o
Return to citation in text: [1] -
Kinder, R. E.; Zhang, Z.; Widenhoefer, R. A. Org. Lett. 2008, 10, 3157. doi:10.1021/ol8010858
Return to citation in text: [1] [2] [3] -
Nishima, N.; Yamamoto, Y. Tetrahedron Lett. 2008, 49, 4908. doi:10.1016/j.tetlet.2008.05.152
Return to citation in text: [1] -
Hannedouche, J.; Aillaud, I.; Collin, J.; Schulz, E.; Trifonov, A. Chem. Commun. 2008, 3552. doi:10.1039/b804745f
Return to citation in text: [1] -
Zi, G.; Xiang, L.; Song, H. Organometallics 2008, 27, 1242. doi:10.1021/om701058k
Return to citation in text: [1] -
Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. J. Am. Chem. Soc. 2007, 129, 14148. doi:10.1021/ja0760731
Return to citation in text: [1] -
LaLonde, R. L.; Sherry, B. D.; Kang, E. J.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 2452. doi:10.1021/ja068819l
Return to citation in text: [1] -
Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. Org. Lett. 2007, 9, 2887. doi:10.1021/ol071108n
Return to citation in text: [1] -
Volz, F.; Krause, N. Org. Biomol. Chem. 2007, 5, 1519. doi:10.1039/b703995f
Return to citation in text: [1] -
Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634. doi:10.1002/ejoc.200600438
Return to citation in text: [1] -
Nishina, N.; Yamamoto, Y. Angew. Chem., Int. Ed. 2006, 45, 3314. doi:10.1002/anie.200600331
Return to citation in text: [1] -
Nishina, N.; Yamamoto, Y. Synlett 2007, 1767. doi:10.1055/s-2007-984501
Return to citation in text: [1] -
Lavallo, V.; Frey, G. D.; Donnadieu, B.; Soleilhavoup, M.; Bertrand, G. Angew. Chem., Int. Ed. 2008, 47, 5224. doi:10.1002/anie.200801136
Return to citation in text: [1] -
Zeng, X.; Soleilhavoup, M.; Bertrand, G. Org. Lett. 2009, 11, 3166. doi:10.1021/ol901418c
Return to citation in text: [1] -
Toups, K. L.; Widenhoefer, R. A. Chem. Commun. 2010, 46, 1712. doi:10.1039/b925859k
Return to citation in text: [1] -
Cooke, M. L.; Xu, K.; Breit, B. Angew. Chem., Int. Ed. 2012, 51, 10876. doi:10.1002/anie.201206594
Return to citation in text: [1] -
Kim, H.; Lim, W.; Im, D.; Kim, D.-G.; Rhee, Y. H. Angew. Chem., Int. Ed. 2012, 51, 12055. doi:10.1002/anie.201206967
Return to citation in text: [1] -
Butler, K. L.; Tragni, M.; Widenhoefer, R. A. Angew. Chem., Int. Ed. 2012, 51, 5175. doi:10.1002/anie.201201584
Return to citation in text: [1] -
Kim, H.; Rhee, Y. H. J. Am. Chem. Soc. 2012, 134, 4011. doi:10.1021/ja2116298
Return to citation in text: [1] -
Al-Masum, M.; Meguro, M.; Yamamoto, Y. Tetrahedron Lett. 1997, 38, 6071. doi:10.1016/S0040-4039(97)01370-1
Return to citation in text: [1] -
Cui, D.-M.; Zheng, Z.-L.; Zhang, C. J. Org. Chem. 2009, 74, 1426. doi:10.1021/jo802513a
Return to citation in text: [1] -
Cui, D.-M.; Yu, K.-R.; Zhang, C. Synlett 2009, 1103. doi:10.1055/s-0028-1088158
Return to citation in text: [1] -
Cui, D.-M.; Zheng, J.-Z.; Yang, L.-Y.; Zhang, C. Synlett 2010, 809. doi:10.1055/s-0029-1219384
Return to citation in text: [1] -
Zhang, Z.; Liu, C.; Kinder, R. E.; Han, X.; Qian, H.; Widenhoefer, R. A. J. Am. Chem. Soc. 2006, 128, 9066. doi:10.1021/ja062045r
Return to citation in text: [1] -
Zhang, Z.; Widenhoefer, R. A. Org. Lett. 2008, 10, 2079. doi:10.1021/ol800646h
Return to citation in text: [1] -
Kennedy-Smith, J. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 4526. doi:10.1021/ja049487s
Return to citation in text: [1] -
Zhang, J.; Yang, C.-G.; He, C. J. Am. Chem. Soc. 2006, 128, 1798. doi:10.1021/ja053864z
Return to citation in text: [1] -
Hashmi, A. S. K.; Weyrauch, J. P.; Frey, W.; Bats, J. W. Org. Lett. 2004, 6, 4391. doi:10.1021/ol0480067
Return to citation in text: [1] -
Liu, Y.; Song, F.; Song, Z.; Liu, M.; Yan, B. Org. Lett. 2005, 7, 5409. doi:10.1021/ol052160r
Return to citation in text: [1] -
Kovács, G.; Ujaque, G.; Lledós, A. J. Am. Chem. Soc. 2008, 130, 853. doi:10.1021/ja073578i
Return to citation in text: [1] -
Wang, Z. J.; Benitez, D.; Tkatchouk, E.; Goddard, W. A., III; Toste, F. D. J. Am. Chem. Soc. 2010, 132, 13064. doi:10.1021/ja105530q
Return to citation in text: [1] -
Searles, S.; Li, Y.; Nassim, B.; Robert Lopes, M.-T.; Tran, P. T.; Crabbé, P. J. Chem. Soc., Perkin Trans. 1 1984, 747. doi:10.1039/p19840000747
Return to citation in text: [1] -
Baird, M. S.; Nizovtsev, A. V.; Bolesov, I. G. Tetrahedron 2002, 58, 1581. doi:10.1016/S0040-4020(02)00018-2
Return to citation in text: [1] -
Régas, D.; Ruiz, J. M.; Afonso, M. M.; Palenzuela, J. A. J. Org. Chem. 2006, 71, 9153. doi:10.1021/jo061582r
Return to citation in text: [1]
| 1. | Müller, T. E.; Hultzsch, K. C.; Yus, F.; Foubelo, M.; Tada, M. Chem. Rev. 2008, 108, 3795. doi:10.1021/cr0306788 |
| 2. | Widenhoefer, R. A.; Han, X. Eur. J. Org. Chem. 2006, 4555. doi:10.1002/ejoc.200600399 |
| 3. | Pohlki, F.; Doye, S. Chem. Soc. Rev. 2003, 32, 104. doi:10.1039/b200386b |
| 4. | Hong, S.; Marks, T. J. Acc. Chem. Res. 2004, 37, 673. doi:10.1021/ar040051r |
| 5. | Hultzsch, K. C. Adv. Synth. Catal. 2005, 347, 367. doi:10.1002/adsc.200404261 |
| 25. | Al-Masum, M.; Meguro, M.; Yamamoto, Y. Tetrahedron Lett. 1997, 38, 6071. doi:10.1016/S0040-4039(97)01370-1 |
| 7. | Kinder, R. E.; Zhang, Z.; Widenhoefer, R. A. Org. Lett. 2008, 10, 3157. doi:10.1021/ol8010858 |
| 16. | Nishina, N.; Yamamoto, Y. Angew. Chem., Int. Ed. 2006, 45, 3314. doi:10.1002/anie.200600331 |
| 17. | Nishina, N.; Yamamoto, Y. Synlett 2007, 1767. doi:10.1055/s-2007-984501 |
| 18. | Lavallo, V.; Frey, G. D.; Donnadieu, B.; Soleilhavoup, M.; Bertrand, G. Angew. Chem., Int. Ed. 2008, 47, 5224. doi:10.1002/anie.200801136 |
| 19. | Zeng, X.; Soleilhavoup, M.; Bertrand, G. Org. Lett. 2009, 11, 3166. doi:10.1021/ol901418c |
| 20. | Toups, K. L.; Widenhoefer, R. A. Chem. Commun. 2010, 46, 1712. doi:10.1039/b925859k |
| 21. | Cooke, M. L.; Xu, K.; Breit, B. Angew. Chem., Int. Ed. 2012, 51, 10876. doi:10.1002/anie.201206594 |
| 22. | Kim, H.; Lim, W.; Im, D.; Kim, D.-G.; Rhee, Y. H. Angew. Chem., Int. Ed. 2012, 51, 12055. doi:10.1002/anie.201206967 |
| 23. | Butler, K. L.; Tragni, M.; Widenhoefer, R. A. Angew. Chem., Int. Ed. 2012, 51, 5175. doi:10.1002/anie.201201584 |
| 24. | Kim, H.; Rhee, Y. H. J. Am. Chem. Soc. 2012, 134, 4011. doi:10.1021/ja2116298 |
| 7. | Kinder, R. E.; Zhang, Z.; Widenhoefer, R. A. Org. Lett. 2008, 10, 3157. doi:10.1021/ol8010858 |
| 8. | Nishima, N.; Yamamoto, Y. Tetrahedron Lett. 2008, 49, 4908. doi:10.1016/j.tetlet.2008.05.152 |
| 9. | Hannedouche, J.; Aillaud, I.; Collin, J.; Schulz, E.; Trifonov, A. Chem. Commun. 2008, 3552. doi:10.1039/b804745f |
| 10. | Zi, G.; Xiang, L.; Song, H. Organometallics 2008, 27, 1242. doi:10.1021/om701058k |
| 11. | Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. J. Am. Chem. Soc. 2007, 129, 14148. doi:10.1021/ja0760731 |
| 12. | LaLonde, R. L.; Sherry, B. D.; Kang, E. J.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 2452. doi:10.1021/ja068819l |
| 13. | Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. Org. Lett. 2007, 9, 2887. doi:10.1021/ol071108n |
| 14. | Volz, F.; Krause, N. Org. Biomol. Chem. 2007, 5, 1519. doi:10.1039/b703995f |
| 15. | Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634. doi:10.1002/ejoc.200600438 |
| 6. | Johannsen, M.; Jørgensen, K. A. Chem. Rev. 1998, 98, 1689. doi:10.1021/cr970343o |
| 37. | Searles, S.; Li, Y.; Nassim, B.; Robert Lopes, M.-T.; Tran, P. T.; Crabbé, P. J. Chem. Soc., Perkin Trans. 1 1984, 747. doi:10.1039/p19840000747 |
| 38. | Baird, M. S.; Nizovtsev, A. V.; Bolesov, I. G. Tetrahedron 2002, 58, 1581. doi:10.1016/S0040-4020(02)00018-2 |
| 39. | Régas, D.; Ruiz, J. M.; Afonso, M. M.; Palenzuela, J. A. J. Org. Chem. 2006, 71, 9153. doi:10.1021/jo061582r |
| 2. | Widenhoefer, R. A.; Han, X. Eur. J. Org. Chem. 2006, 4555. doi:10.1002/ejoc.200600399 |
| 7. | Kinder, R. E.; Zhang, Z.; Widenhoefer, R. A. Org. Lett. 2008, 10, 3157. doi:10.1021/ol8010858 |
| 29. | Zhang, Z.; Liu, C.; Kinder, R. E.; Han, X.; Qian, H.; Widenhoefer, R. A. J. Am. Chem. Soc. 2006, 128, 9066. doi:10.1021/ja062045r |
| 30. | Zhang, Z.; Widenhoefer, R. A. Org. Lett. 2008, 10, 2079. doi:10.1021/ol800646h |
| 31. | Kennedy-Smith, J. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 4526. doi:10.1021/ja049487s |
| 32. | Zhang, J.; Yang, C.-G.; He, C. J. Am. Chem. Soc. 2006, 128, 1798. doi:10.1021/ja053864z |
| 33. | Hashmi, A. S. K.; Weyrauch, J. P.; Frey, W.; Bats, J. W. Org. Lett. 2004, 6, 4391. doi:10.1021/ol0480067 |
| 34. | Liu, Y.; Song, F.; Song, Z.; Liu, M.; Yan, B. Org. Lett. 2005, 7, 5409. doi:10.1021/ol052160r |
| 35. | Kovács, G.; Ujaque, G.; Lledós, A. J. Am. Chem. Soc. 2008, 130, 853. doi:10.1021/ja073578i |
| 36. | Wang, Z. J.; Benitez, D.; Tkatchouk, E.; Goddard, W. A., III; Toste, F. D. J. Am. Chem. Soc. 2010, 132, 13064. doi:10.1021/ja105530q |
| 26. | Cui, D.-M.; Zheng, Z.-L.; Zhang, C. J. Org. Chem. 2009, 74, 1426. doi:10.1021/jo802513a |
| 27. | Cui, D.-M.; Yu, K.-R.; Zhang, C. Synlett 2009, 1103. doi:10.1055/s-0028-1088158 |
| 28. | Cui, D.-M.; Zheng, J.-Z.; Yang, L.-Y.; Zhang, C. Synlett 2010, 809. doi:10.1055/s-0029-1219384 |
© 2013 Zhang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)




![[1860-5397-9-117-1]](/bjoc/content/figures/1860-5397-9-117-1.png?scale=2.0&max-width=1024&background=FFFFFF)
![[1860-5397-9-117-2]](/bjoc/content/figures/1860-5397-9-117-2.png?scale=2.0&max-width=1024&background=FFFFFF)