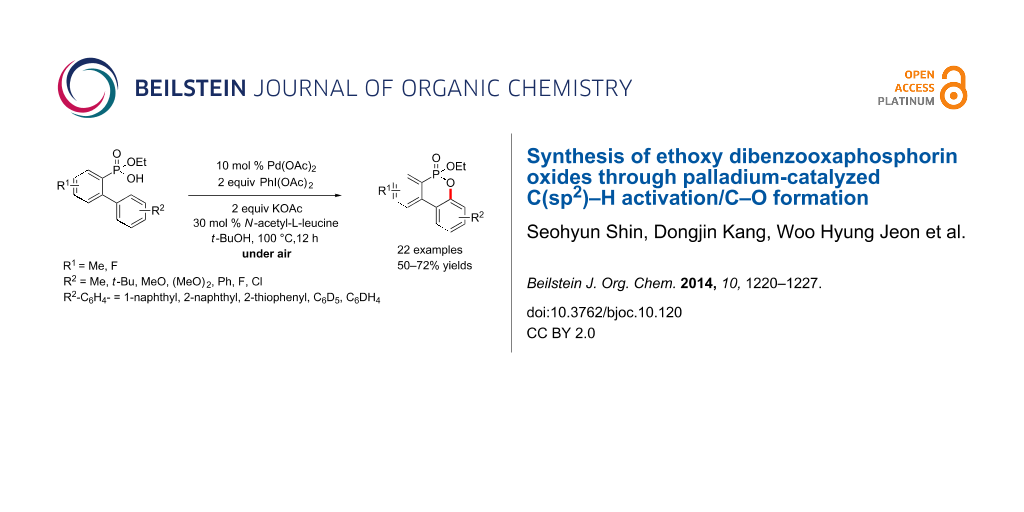
Abstract
We report an efficient Pd-catalyzed C(sp2)–H activation/C–O bond formation for the synthesis of ethoxy dibenzooxaphosphorin oxides from 2-(aryl)arylphosphonic acid monoethyl esters under aerobic conditions.
Graphical Abstract
Introduction
Unreactive C(sp2)–H and C(sp3)–H bonds are ubiquitous in organic compounds [1-7], so that the development of methods for the transition metal-catalyzed C–H activation is one of the challenging goals in organic synthesis. Especially, the development of synthetic methods of C–heteroatom bond formation via C–H activation has received attention owing to the omnipresence of heterocyclic compounds in nature [8]. Recently, it has been demonstrated that the intramolecular bond formation between a heteroatom and a vicinal unreactive C–H is an efficient method for the synthesis of heterocycles [9-17]. Although C–H activation/C–N formation has been widely used for the synthesis of azaheterocycles, the preparation of oxaheterocycles via C–H activation/C–O formation has been described a lot less, because the energy correlation between the HOMO of the Pd–O bond and the LUMO of the Pd–C bond is unfavorable and the Pd–O bond has a significantly ionic character [18-23]. To expand this scope, we are interested in the development of C–H activation/C–O formation by means of new directing groups. Recently, a variety of C–H activations by using new phosphoryl-related directing groups have been reported by our [24-32] and other groups [33-41]. More recently, we developed a method allowing for synthetic access to benzoxaphosphole 1- and 2-oxides starting from phosphonic and phosphinic acids via Pd-catalyzed C(sp2 and sp3)–H activation/C–O formation [42]. In this context, we herein report the synthetic method of alkoxy dibenzooxaphosphorin oxides from 2-(aryl)arylphosphonic acid monoesters via Pd-catalyzed C(sp2)–H activation/C–O formation (Scheme 1).
Scheme 1: Synthesis of alkoxy dibenzooxaphosphorin oxides by C(sp2)–H activation/C–O formation.
Scheme 1: Synthesis of alkoxy dibenzooxaphosphorin oxides by C(sp2)–H activation/C–O formation.
Results and Discussion
First, a wide range of 2-(aryl)arylphosphonic acid monoethyl esters were efficiently prepared by a Suzuki reaction of 2-bromoiodoarenes with arylboronic acids, a lithium bromide exchange reaction of 2-bromobiaryls followed by diethylphosphinylation with diethyl chlorophosphate, and the C–O cleavage of diethyl 2-(aryl)arylphosphonates by using L-Selectride (Scheme 2).
Scheme 2: Preparation of 2-(aryl)arylphosphonic acid monoethyl esters.
Scheme 2: Preparation of 2-(aryl)arylphosphonic acid monoethyl esters.
The C–H activation/C–O formation of 2-(phenyl)phenylphosphonic acid monoethyl ester (1a) was examined with a variety of oxidants and bases in the presence of Pd(OAc)2. A multitude of oxidants such as K2S2O8, BQ, benzoyl peroxide, PhI(TFA)2, Cu(OAc)2, CuCl2, CuBr, AgOAc, Ag2CO3 and Ag2O did not produce the cyclized product 2a (see Supporting Information File 1). However, PhI(OAc)2, which is an efficient oxidant for the Pd(II)/Pd(IV) catalytic cycle, gave 2a in 30% yield in t-butanol (80 °C for 16 h; Table 1, entry 1) [19,43-47]. In addition, various bases were examined. Although NaOAc, CsOAc, CsF and CsOPiv afforded 2a in yields ranging from 42% to 52%, KOAc gave the best result (57%) in the presence of PhI(OAc)2 in tert-butanol (see Supporting Information File 1). tert-Butanol gave the best result among the solvents DCE, dioxane, ACN, t-AmOH, DMF, HFIP, THF, toluene, TFA and MeOH (see Supporting Information File 1). With this preliminary result in hand, we investigated a variety of organic acids as ligands in an effort to improve the catalytic efficiency (Scheme 3). However, these attempts provided no improvement (Table 1, entries 2–4). Finally, we discovered that easily accessible monoprotected amino acids, which have recently been established as efficient ligands in C–H activations [48-50], increased the yield (Table 1, entries 5–10). Among the investigated ligands, N-acetyl-L-leucine (L9) gave the best results (Table 1, entry 10). After examination of the reaction temperature (Table 1, entries 11–13) and time (Table 1, entries 14–16), the oxidative cyclization using PhI(OAc)2 (2 equiv) and KOAc (2 equiv) in the presence of Pd(OAc)2 (10 mol %) and L9 (30 mol %) gave the best result under aerobic conditions, affording 2a in 61% yield (isolated yield 55%, Table 1, entry 16). Both Pd(TFA)2 and Pd(OTf)2∙H2O gave inferior results compared to Pd(OAc)2 (Table 1, entries 17 and 18).
Table 1: Optimization studies for the cyclization of 2-(phenyl)phenylphosphonic acid monoethyl esters.
|
|
|||||
| entry | cat. Pd | ligand | T [°C] | t [h] | yielda [%] |
|---|---|---|---|---|---|
| 1 | 10 mol % Pd(OAc)2 | — | 80 | 16 | 30 |
| 2 | 10 mol % Pd(OAc)2 | 30 mol % L1 | 80 | 16 | 23 |
| 3 | 10 mol % Pd(OAc)2 | 30 mol % L2 | 80 | 16 | 34 |
| 4 | 10 mol % Pd(OAc)2 | 30 mol % L3 | 80 | 16 | 28 |
| 5 | 10 mol % Pd(OAc)2 | 30 mol % L4 | 80 | 16 | 48 |
| 6 | 10 mol % Pd(OAc)2 | 30 mol % L5 | 80 | 16 | 48 |
| 7 | 10 mol % Pd(OAc)2 | 30 mol % L6 | 80 | 16 | 54 |
| 8 | 10 mol % Pd(OAc)2 | 30 mol % L7 | 80 | 16 | 53 |
| 9 | 10 mol % Pd(OAc)2 | 30 mol % L8 | 80 | 16 | 51 |
| 10 | 10 mol % Pd(OAc)2 | 30 mol % L9 | 80 | 16 | 57 |
| 11 | 10 mol % Pd(OAc)2 | 30 mol % L9 | 60 | 16 | 20 |
| 12 | 10 mol % Pd(OAc)2 | 30 mol % L9 | 100 | 16 | 61 |
| 13 | 10 mol % Pd(OAc)2 | 30 mol % L9 | 120 | 16 | 50 |
| 14 | 10 mol % Pd(OAc)2 | 30 mol % L9 | 100 | 4 | 45 |
| 15 | 10 mol % Pd(OAc)2 | 30 mol % L9 | 100 | 8 | 51 |
| 16 | 10 mol % Pd(OAc)2 | 30 mol % L9 | 100 | 12 | 61(55) |
| 17 | 10 mol % Pd(TFA)2 | 30 mol % L9 | 100 | 12 | 53 |
| 18 | 10 mol % Pd(OTf)2·H2O | 30 mol % L9 | 100 | 12 | 45 |
aYields were determined by 1H NMR with CH2Br2 as an internal standard. The number in parentheses is the isolated yield.
Scheme 3: A variety of organic acids and monoprotected amino acids as ligands.
Scheme 3: A variety of organic acids and monoprotected amino acids as ligands.
To ascertain the scope of the Pd-catalyzed C–H activation followed by the C–O formation, a wide range of 2-(aryl)phenylphosphonic acid monoethyl esters 1 were examined under the optimized reaction conditions (Scheme 4). Phenylphosphonic acid monoethyl ester 1b with a 2-methyl group on the phenyl ring was transformed to the desired dibenzooxaphosphorin oxide 2b in 53% yield. Phenylphosphonic acid monoethyl esters (1c) with a 3-methyl group were selectively converted to the cyclized products (2c) in 66% yield due to steric effects. In the case of 4-tert-butyl, the desired product 2e was obtained in 65% yield. Substrate 1f, characterized by an electron-donating 4-methoxy group, was cyclized to dibenzooxaphosphorin oxide 2f in 65% yield under aerobic conditions. The present method worked equally well with 3,4-dimethoxyphenyl-substituted phenylphosphonic acid monoethyl ester 1g. Phenylphosphonic acid monoethyl ester 1h with a 4-phenyl group on the phenyl ring turned out to be compatible with the reaction conditions. As anticipated, 2-naphthyl-substituted phenylphosphonic acid monoethyl ester 1i underwent the Pd-catalyzed oxidative cyclization regioselectively at the sterically less hindered position to afford the desired dibenzooxaphosphorin oxide 2i in 70% yield. We were pleased to obtain 2j by a Pd-catalyzed oxidative cyclization of 1-naphthyl-substituted phenylphosphonic acid monoethyl ester 1j. 2-(Aryl)phenylphosphonic acid monoethyl esters 1k, 1l and 1m with an electron-withdrawing fluoro or chloro group on the phenyl ring were subjected to the oxidative cyclization to deliver the desired products 2k, 2l and 2m in yields ranging from 54% and 64%. In particular, the tolerance of the chloro groups may be of importance for a subsequent catalytic cross-coupling reaction. Substrate 1n, which contains a 2-thiophenyl moiety, was subjected to the cyclization affording 2n in 52% yield. The preparation of 2-arylphenylphosphonic acid monoethyl esters with a nitro, difluoro, or ethoxycarbonyl group failed.
Scheme 4: Cyclization of 2-arylphenylphosphonic acid monoethyl esters.
Scheme 4: Cyclization of 2-arylphenylphosphonic acid monoethyl esters.
Next, the Pd-catalyzed oxidative cyclization of 2-(aryl)arylphosphonic acid monoethyl esters 3 were examined to demonstrate the efficiency of the present method (Scheme 5). 4-Methylphenylphosphonic acid monoethyl esters 3a and 3b with a 3-methyl- and 3,4-dimethoxyphenyl group at 2-position turned out to be compatible with the Pd-catalyzed oxidative cyclization. There are no regioisomers formed due to steric effects. Substrate 3c bearing a chloro group was selectively cyclized to afford 4c in 64% yield. To our delight, the present method worked equally well even if a fluoro group on the phenyl ring is present. 3-Fluorophenylphosphonic acid monoethyl esters 3d, 3e and 3f with 3-methyl-, 3,4-dimethoxy and 3-chlorophenyl groups at the 2-position selectively underwent the oxidative cyclization to give the corresponding cyclized products 4d, 4e and 4f in yields ranging from 50% and 63%.
Scheme 5: Cyclization of 2-(aryl)arylphosphonic acid monoethyl esters.
Scheme 5: Cyclization of 2-(aryl)arylphosphonic acid monoethyl esters.
We carried out kinetic isotope effect (KIE) studies to prove the reaction mechanism (see Scheme 8). The required deuterium-labeled 2-(phenyl)phenylphosphonic acid monoethyl ester 1a-[D5] was efficiently prepared by a Suzuki reaction of deuterated bromobenzene (6) with 2-bromophenylboronic acid (5), a lithium bromide exchange reaction of 2-bromo deuterated biphenyl 7 followed by diethylphosphinylation with diethyl chlorophosphate, and C–O cleavage of diethyl 2-(phenyl)phenylphosphonate by using L-Selectride (Scheme 6). In addition, the deuterium-labeled 2-(phenyl)phenylphosphonic acid monoethyl ester 1a-[D1] was obtained by the lithium bromide exchange reaction of 2‘-bromo-2-iodo-1,1‘-biphenyl (10) and the treatment of D2O, diethylphosphinylation with diethyl chlorophosphate, and C–O cleavage of diethyl 2-(phenyl)phenylphosphonate by using L-Selectride (Scheme 7).
In the case of an intermolecular competition reaction using 1a and 1a-[D5], a KIE was detected (kH/kD = 1.0; Scheme 8, reaction 1) [51,52]. Also, an intramolecular competition reaction using 1a-[D1] was carried out to give KIE (kH/kD = 0.6; Scheme 8, reaction 2). These results indicate that the C–H cleavage at the ortho-position of 2-(phenyl)phenylphosphonic acid monoethyl ester is not involved in the rate-limiting step and the C–H bond metallation is reversible.
Scheme 8: Studies with isotopically labelled compounds.
Scheme 8: Studies with isotopically labelled compounds.
To elucidate the mechanism of the present reaction, the reaction was conducted with a stoichiometric amount of Pd(OAc)2 and without the oxidant PhI(OAc)2. However, no cyclized product was observed. This result indicates that the C–O reductive elimination from Pd(II) is not favorable. Because both the intermolecular and intramolecular competition experiments exhibited no significant kinetic isotope effect (kH/kD = 1.0 and 0.6; Scheme 8), we hypothesize that the C–O reductive elimination step is the rate-determining step. A feasible mechanism involving the Pd(II)/Pd(IV) catalytic cycle is described in Scheme 9. The C–H activation might be efficiently accelerated by the N–H activation propelled by N-Ac-L-Leu-OH (L9) as a ligand [53-55], resulting in the formation of palladacycle III. Thereafter, ethoxy dibenzooxaphosphorin oxide 2a is obtained from the oxidation of the Pd(II) to Pd(IV) species IV and the subsequent C–O reductive elimination.
Conclusion
In this paper, we have developed an efficient synthetic method for a wide range of ethoxy dibenzooxaphosphorin oxides starting from 2-(aryl)arylphosphonic acid monoethyl esters and employing Pd-catalyzed C(sp2)–H activation/C–O formation under aerobic conditions. Oxidative cyclization by means of a Pd(II)/Pd(IV) catalytic cycle might play a role in the mechanism of the present reaction.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, and 1H and 13C NMR spectra of new compounds. | ||
| Format: PDF | Size: 3.6 MB | Download |
References
-
Murai, S.; Alper, H.; Gossage, R. A.; Grushin, V. V.; Hidai, M.; Ito, Y.; Jones, W. D.; Kakiuchi, F.; van Koten, G.; Lin, Y.-S.; Mizobe, Y.; Murai, S.; Murakami, M.; Richmond, T. G.; Sen, A.; Suginome, M.; Yamamoto, A., Eds. Activation of Unreactive Bonds and Organic Synthesis; Springer: Berlin, 1999. doi:10.1007/3-540-68525-1
Return to citation in text: [1] -
Dyker, G., Ed. Handbook of C–H Transformations; Wiley-VCH: Weinheim, 2005. doi:10.1002/9783527619450
Return to citation in text: [1] -
Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147. doi:10.1021/cr900184e
Return to citation in text: [1] -
Colby, D. A.; Bergman, R. G.; Ellman, J. A. Chem. Rev. 2010, 110, 624. doi:10.1021/cr900005n
Return to citation in text: [1] -
Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293. doi:10.1021/cr100198w
Return to citation in text: [1] -
Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068. doi:10.1039/c1cs15082k
Return to citation in text: [1] -
Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215. doi:10.1021/cr100280d
Return to citation in text: [1] -
Li, J.-J.; Corey, E. J., Eds. Name Reactions in Heterocyclic Chemistry; Wiley-Interscience: Hoboken, NJ, 2005. doi:10.1002/0471704156
Return to citation in text: [1] -
Thansandote, P.; Lautens, M. Chem.–Eur. J. 2009, 15, 5874. doi:10.1002/chem.200900281
Return to citation in text: [1] -
Espino, C. G.; Wehn, P. M.; Chow, J.; Du Bois, J. J. Am. Chem. Soc. 2001, 123, 6935. doi:10.1021/ja011033x
Return to citation in text: [1] -
Liang, J.-L.; Yuan, S.-X.; Huang, J.-S.; Che, C.-M. J. Org. Chem. 2004, 69, 3610. doi:10.1021/jo0358877
Return to citation in text: [1] -
Jordan-Hore, J. A.; Johansson, C. C. C.; Gulias, M.; Beck, E. M.; Gaunt, M. J. J. Am. Chem. Soc. 2008, 130, 16184. doi:10.1021/ja806543s
Return to citation in text: [1] -
Mei, T.-S.; Wang, X.; Yu, J.-Q. J. Am. Chem. Soc. 2009, 131, 10806. doi:10.1021/ja904709b
Return to citation in text: [1] -
Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2008, 130, 14058. doi:10.1021/ja807129e
Return to citation in text: [1] -
Neumann, J. J.; Rakshit, S.; Dröge, T.; Glorius, F. Angew. Chem., Int. Ed. 2009, 48, 6892. doi:10.1002/anie.200903035
Return to citation in text: [1] -
Inamoto, K.; Saito, T.; Katsuno, M.; Sakamoto, T.; Hiroya, K. Org. Lett. 2007, 9, 2931. doi:10.1021/ol0711117
Return to citation in text: [1] -
Wang, H.; Wang, Y.; Peng, C.; Zhang, J.; Zhu, Q. J. Am. Chem. Soc. 2010, 132, 13217. doi:10.1021/ja1067993
Return to citation in text: [1] -
Hartwig, J. F. Inorg. Chem. 2007, 46, 1936. doi:10.1021/ic061926w
Return to citation in text: [1] -
Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300. doi:10.1021/ja031543m
Return to citation in text: [1] [2] -
Novák, P.; Correa, A.; Gallardo-Donaire, J.; Martin, R. Angew. Chem., Int. Ed. 2011, 50, 12236. doi:10.1002/anie.201105894
Return to citation in text: [1] -
Gallardo-Donaire, J.; Martin, R. J. Am. Chem. Soc. 2013, 135, 9350. doi:10.1021/ja4047894
Return to citation in text: [1] -
Cheng, X.-F.; Li, Y.; Su, Y.-M.; Yin, F.; Wang, J.-Y.; Sheng, J.; Vora, H. U.; Wang, X.-S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 1236. doi:10.1021/ja311259x
Return to citation in text: [1] -
Yang, M.; Jiang, X.; Shi, W.-J.; Zhu, Q.-L.; Shi, Z.-J. Org. Lett. 2013, 15, 690. doi:10.1021/ol303569b
Return to citation in text: [1] -
Seo, J.; Park, Y.; Jeon, I.; Ryu, T.; Park, S.; Lee, P. H. Org. Lett. 2013, 15, 3358. doi:10.1021/ol401407v
Return to citation in text: [1] -
Chary, B. C.; Kim, S.; Park, Y.; Kim, J.; Lee, P. H. Org. Lett. 2013, 15, 2692. doi:10.1021/ol4009987
Return to citation in text: [1] -
Chan, L. Y.; Kim, S.; Ryu, T.; Lee, P. H. Chem. Commun. 2013, 49, 4682. doi:10.1039/c3cc41107a
Return to citation in text: [1] -
Ryu, T.; Kim, J.; Park, Y.; Kim, S.; Lee, P. H. Org. Lett. 2013, 15, 3986. doi:10.1021/ol401775t
Return to citation in text: [1] -
Park, S.; Seo, B.; Shin, S.; Son, J.-Y.; Lee, P. H. Chem. Commun. 2013, 49, 8671. doi:10.1039/c3cc44995e
Return to citation in text: [1] -
Mo, J.; Lim, S.; Park, S.; Ryu, T.; Kim, S.; Lee, P. H. RSC Adv. 2013, 3, 18296. doi:10.1039/c3ra43764g
Return to citation in text: [1] -
Park, Y.; Seo, J.; Park, S.; Yoo, E. J.; Lee, P. H. Chem.–Eur. J. 2013, 19, 16461. doi:10.1002/chem.201302652
Return to citation in text: [1] -
Park, Y.; Jeon, I.; Shin, S.; Min, J.; Lee, P. H. J. Org. Chem. 2013, 78, 10209. doi:10.1021/jo401608v
Return to citation in text: [1] -
Kang, D.; Cho, J.; Lee, P. H. Chem. Commun. 2013, 49, 10501. doi:10.1039/c3cc45874a
Return to citation in text: [1] -
Meng, X.; Kim, S. Org. Lett. 2013, 15, 1910. doi:10.1021/ol400565r
Return to citation in text: [1] -
Chan, L. Y.; Cheong, L.; Kim, S. Org. Lett. 2013, 15, 2186. doi:10.1021/ol400732q
Return to citation in text: [1] -
Jeon, W. H.; Lee, T. S.; Kim, E. J.; Moon, B.; Kang, J. Tetrahedron 2013, 69, 5152. doi:10.1016/j.tet.2013.04.067
Return to citation in text: [1] -
Hu, R.-B.; Zhang, H.; Zhang, X.-Y.; Yang, S.-D. Chem. Commun. 2014, 50, 2193. doi:10.1039/c3cc49050e
Return to citation in text: [1] -
Wang, H.-L.; Hu, R.-B.; Zhang, H.; Zou, A.-X.; Yang, S.-D. Org. Lett. 2013, 15, 5302. doi:10.1021/ol402577p
Return to citation in text: [1] -
Zhang, H.-Y.; Yi, H.-M.; Wang, G.-W.; Yang, B.; Yang, S.-D. Org. Lett. 2013, 15, 6186. doi:10.1021/ol403028a
Return to citation in text: [1] -
Unoh, Y.; Hashimoto, Y.; Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2013, 15, 3258. doi:10.1021/ol4012794
Return to citation in text: [1] -
Itoh, M.; Hashimoto, Y.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2013, 78, 8098. doi:10.1021/jo401393b
Return to citation in text: [1] -
Zhao, D.; Nimphius, C.; Lindale, M.; Glorius, F. Org. Lett. 2013, 15, 4504. doi:10.1021/ol402053n
Return to citation in text: [1] -
Eom, D.; Jeong, Y.; Kim, Y. R.; Lee, E.; Choi, W.; Lee, P. H. Org. Lett. 2013, 15, 5210. doi:10.1021/ol402736v
Return to citation in text: [1] -
Yoneyama, T.; Crabtree, R. H. J. Mol. Catal. A: Chem. 1996, 108, 35. doi:10.1016/1381-1169(95)00289-8
Return to citation in text: [1] -
Muñiz, K. Angew. Chem., Int. Ed. 2009, 48, 9412. doi:10.1002/anie.200903671
Return to citation in text: [1] -
Wei, Y.; Yoshikai, N. Org. Lett. 2011, 13, 5504. doi:10.1021/ol202229w
Return to citation in text: [1] -
Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313. doi:10.1021/ja3023972
Return to citation in text: [1] -
Hickman, A. J.; Sanford, M. S. Nature 2012, 484, 177. doi:10.1038/nature11008
Return to citation in text: [1] -
Wang, D.-H.; Engle, K. M.; Shi, B.-F.; Yu, J.-Q. Science 2010, 327, 315. doi:10.1126/science.1182512
Return to citation in text: [1] -
Lu, Y.; Wang, D.-H.; Engle, K. M.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 5916. doi:10.1021/ja101909t
Return to citation in text: [1] -
Cheng, G.-J.; Yang, Y.-F.; Liu, P.; Chen, P.; Sun, T.-Y.; Li, G.; Zhang, X.; Houk, K. N.; Yu, J.-Q.; Wu, W.-D. J. Am. Chem. Soc. 2014, 136, 894. doi:10.1021/ja411683n
Return to citation in text: [1] -
Jones, W. D. Acc. Chem. Res. 2003, 36, 140. doi:10.1021/ar020148i
Return to citation in text: [1] -
Kakiuchi, F.; Matsuura, Y.; Kan, S.; Chatani, N. J. Am. Chem. Soc. 2005, 127, 5936. doi:10.1021/ja043334n
Return to citation in text: [1] -
Musaev, D. G.; Kaledin, A. L.; Shi, B.-F.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 1690. doi:10.1021/ja208661v
Return to citation in text: [1] -
Baxter, R. D.; Sale, D.; Engle, K. M.; Yu, J.-Q.; Blackmond, D. G. J. Am. Chem. Soc. 2012, 134, 4600. doi:10.1021/ja207634t
Return to citation in text: [1] -
Li, Y.; Ding, Y.-J.; Wang, J.-Y.; Su, Y.-M.; Wang, X.-S. Org. Lett. 2013, 15, 2574. doi:10.1021/ol400877q
Return to citation in text: [1]
| 1. | Murai, S.; Alper, H.; Gossage, R. A.; Grushin, V. V.; Hidai, M.; Ito, Y.; Jones, W. D.; Kakiuchi, F.; van Koten, G.; Lin, Y.-S.; Mizobe, Y.; Murai, S.; Murakami, M.; Richmond, T. G.; Sen, A.; Suginome, M.; Yamamoto, A., Eds. Activation of Unreactive Bonds and Organic Synthesis; Springer: Berlin, 1999. doi:10.1007/3-540-68525-1 |
| 2. | Dyker, G., Ed. Handbook of C–H Transformations; Wiley-VCH: Weinheim, 2005. doi:10.1002/9783527619450 |
| 3. | Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147. doi:10.1021/cr900184e |
| 4. | Colby, D. A.; Bergman, R. G.; Ellman, J. A. Chem. Rev. 2010, 110, 624. doi:10.1021/cr900005n |
| 5. | Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293. doi:10.1021/cr100198w |
| 6. | Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068. doi:10.1039/c1cs15082k |
| 7. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215. doi:10.1021/cr100280d |
| 24. | Seo, J.; Park, Y.; Jeon, I.; Ryu, T.; Park, S.; Lee, P. H. Org. Lett. 2013, 15, 3358. doi:10.1021/ol401407v |
| 25. | Chary, B. C.; Kim, S.; Park, Y.; Kim, J.; Lee, P. H. Org. Lett. 2013, 15, 2692. doi:10.1021/ol4009987 |
| 26. | Chan, L. Y.; Kim, S.; Ryu, T.; Lee, P. H. Chem. Commun. 2013, 49, 4682. doi:10.1039/c3cc41107a |
| 27. | Ryu, T.; Kim, J.; Park, Y.; Kim, S.; Lee, P. H. Org. Lett. 2013, 15, 3986. doi:10.1021/ol401775t |
| 28. | Park, S.; Seo, B.; Shin, S.; Son, J.-Y.; Lee, P. H. Chem. Commun. 2013, 49, 8671. doi:10.1039/c3cc44995e |
| 29. | Mo, J.; Lim, S.; Park, S.; Ryu, T.; Kim, S.; Lee, P. H. RSC Adv. 2013, 3, 18296. doi:10.1039/c3ra43764g |
| 30. | Park, Y.; Seo, J.; Park, S.; Yoo, E. J.; Lee, P. H. Chem.–Eur. J. 2013, 19, 16461. doi:10.1002/chem.201302652 |
| 31. | Park, Y.; Jeon, I.; Shin, S.; Min, J.; Lee, P. H. J. Org. Chem. 2013, 78, 10209. doi:10.1021/jo401608v |
| 32. | Kang, D.; Cho, J.; Lee, P. H. Chem. Commun. 2013, 49, 10501. doi:10.1039/c3cc45874a |
| 18. | Hartwig, J. F. Inorg. Chem. 2007, 46, 1936. doi:10.1021/ic061926w |
| 19. | Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300. doi:10.1021/ja031543m |
| 20. | Novák, P.; Correa, A.; Gallardo-Donaire, J.; Martin, R. Angew. Chem., Int. Ed. 2011, 50, 12236. doi:10.1002/anie.201105894 |
| 21. | Gallardo-Donaire, J.; Martin, R. J. Am. Chem. Soc. 2013, 135, 9350. doi:10.1021/ja4047894 |
| 22. | Cheng, X.-F.; Li, Y.; Su, Y.-M.; Yin, F.; Wang, J.-Y.; Sheng, J.; Vora, H. U.; Wang, X.-S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 1236. doi:10.1021/ja311259x |
| 23. | Yang, M.; Jiang, X.; Shi, W.-J.; Zhu, Q.-L.; Shi, Z.-J. Org. Lett. 2013, 15, 690. doi:10.1021/ol303569b |
| 9. | Thansandote, P.; Lautens, M. Chem.–Eur. J. 2009, 15, 5874. doi:10.1002/chem.200900281 |
| 10. | Espino, C. G.; Wehn, P. M.; Chow, J.; Du Bois, J. J. Am. Chem. Soc. 2001, 123, 6935. doi:10.1021/ja011033x |
| 11. | Liang, J.-L.; Yuan, S.-X.; Huang, J.-S.; Che, C.-M. J. Org. Chem. 2004, 69, 3610. doi:10.1021/jo0358877 |
| 12. | Jordan-Hore, J. A.; Johansson, C. C. C.; Gulias, M.; Beck, E. M.; Gaunt, M. J. J. Am. Chem. Soc. 2008, 130, 16184. doi:10.1021/ja806543s |
| 13. | Mei, T.-S.; Wang, X.; Yu, J.-Q. J. Am. Chem. Soc. 2009, 131, 10806. doi:10.1021/ja904709b |
| 14. | Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2008, 130, 14058. doi:10.1021/ja807129e |
| 15. | Neumann, J. J.; Rakshit, S.; Dröge, T.; Glorius, F. Angew. Chem., Int. Ed. 2009, 48, 6892. doi:10.1002/anie.200903035 |
| 16. | Inamoto, K.; Saito, T.; Katsuno, M.; Sakamoto, T.; Hiroya, K. Org. Lett. 2007, 9, 2931. doi:10.1021/ol0711117 |
| 17. | Wang, H.; Wang, Y.; Peng, C.; Zhang, J.; Zhu, Q. J. Am. Chem. Soc. 2010, 132, 13217. doi:10.1021/ja1067993 |
| 8. | Li, J.-J.; Corey, E. J., Eds. Name Reactions in Heterocyclic Chemistry; Wiley-Interscience: Hoboken, NJ, 2005. doi:10.1002/0471704156 |
| 48. | Wang, D.-H.; Engle, K. M.; Shi, B.-F.; Yu, J.-Q. Science 2010, 327, 315. doi:10.1126/science.1182512 |
| 49. | Lu, Y.; Wang, D.-H.; Engle, K. M.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 5916. doi:10.1021/ja101909t |
| 50. | Cheng, G.-J.; Yang, Y.-F.; Liu, P.; Chen, P.; Sun, T.-Y.; Li, G.; Zhang, X.; Houk, K. N.; Yu, J.-Q.; Wu, W.-D. J. Am. Chem. Soc. 2014, 136, 894. doi:10.1021/ja411683n |
| 53. | Musaev, D. G.; Kaledin, A. L.; Shi, B.-F.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 1690. doi:10.1021/ja208661v |
| 54. | Baxter, R. D.; Sale, D.; Engle, K. M.; Yu, J.-Q.; Blackmond, D. G. J. Am. Chem. Soc. 2012, 134, 4600. doi:10.1021/ja207634t |
| 55. | Li, Y.; Ding, Y.-J.; Wang, J.-Y.; Su, Y.-M.; Wang, X.-S. Org. Lett. 2013, 15, 2574. doi:10.1021/ol400877q |
| 19. | Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300. doi:10.1021/ja031543m |
| 43. | Yoneyama, T.; Crabtree, R. H. J. Mol. Catal. A: Chem. 1996, 108, 35. doi:10.1016/1381-1169(95)00289-8 |
| 44. | Muñiz, K. Angew. Chem., Int. Ed. 2009, 48, 9412. doi:10.1002/anie.200903671 |
| 45. | Wei, Y.; Yoshikai, N. Org. Lett. 2011, 13, 5504. doi:10.1021/ol202229w |
| 46. | Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313. doi:10.1021/ja3023972 |
| 47. | Hickman, A. J.; Sanford, M. S. Nature 2012, 484, 177. doi:10.1038/nature11008 |
| 42. | Eom, D.; Jeong, Y.; Kim, Y. R.; Lee, E.; Choi, W.; Lee, P. H. Org. Lett. 2013, 15, 5210. doi:10.1021/ol402736v |
| 33. | Meng, X.; Kim, S. Org. Lett. 2013, 15, 1910. doi:10.1021/ol400565r |
| 34. | Chan, L. Y.; Cheong, L.; Kim, S. Org. Lett. 2013, 15, 2186. doi:10.1021/ol400732q |
| 35. | Jeon, W. H.; Lee, T. S.; Kim, E. J.; Moon, B.; Kang, J. Tetrahedron 2013, 69, 5152. doi:10.1016/j.tet.2013.04.067 |
| 36. | Hu, R.-B.; Zhang, H.; Zhang, X.-Y.; Yang, S.-D. Chem. Commun. 2014, 50, 2193. doi:10.1039/c3cc49050e |
| 37. | Wang, H.-L.; Hu, R.-B.; Zhang, H.; Zou, A.-X.; Yang, S.-D. Org. Lett. 2013, 15, 5302. doi:10.1021/ol402577p |
| 38. | Zhang, H.-Y.; Yi, H.-M.; Wang, G.-W.; Yang, B.; Yang, S.-D. Org. Lett. 2013, 15, 6186. doi:10.1021/ol403028a |
| 39. | Unoh, Y.; Hashimoto, Y.; Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2013, 15, 3258. doi:10.1021/ol4012794 |
| 40. | Itoh, M.; Hashimoto, Y.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2013, 78, 8098. doi:10.1021/jo401393b |
| 41. | Zhao, D.; Nimphius, C.; Lindale, M.; Glorius, F. Org. Lett. 2013, 15, 4504. doi:10.1021/ol402053n |
| 51. | Jones, W. D. Acc. Chem. Res. 2003, 36, 140. doi:10.1021/ar020148i |
| 52. | Kakiuchi, F.; Matsuura, Y.; Kan, S.; Chatani, N. J. Am. Chem. Soc. 2005, 127, 5936. doi:10.1021/ja043334n |
© 2014 Shin et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)