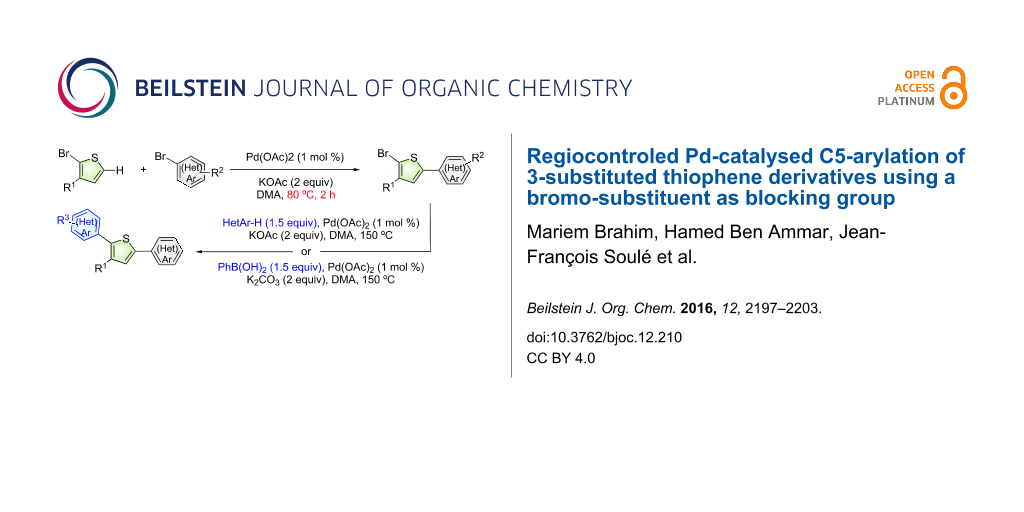
Abstract
The use of a bromo-substituent as blocking group at the C2-position of 3-substituted thiophenes allows the regioselective introduction of aryl substituents at C5-position via Pd-catalysed direct arylation. With 1 mol % of a phosphine-free Pd catalyst, KOAc as the base and DMA as the solvent and various electron-deficient aryl bromides as aryl sources, C5-(hetero)arylated thiophenes were synthesized in moderate to high yields, without cleavage of the thienyl C–Br bond. Moreover, sequential direct thienyl C5-arylation followed by Pd-catalysed direct arylation or Suzuki coupling at the C2-position allows to prepare 2,5-di(hetero)arylated thiophenes bearing two different (hetero)aryl units in only two steps. This method provides a “green” access to arylated thiophene derivatives as it reduces the number of steps to prepare these compounds and also the formation of wastes.
Graphical Abstract
Introduction
Thiophene derivatives bearing aryl substituents are important structures because of their biological and/or physical properties. Among them, 3-substituted 5-arylthiophenes are widely used as building blocks for the synthesis of semi-conductors [1-3]. Therefore, the discovery of more direct and selective procedures for access to 5-arylated 3-substituted thiophene derivatives is an important topic in sustainable chemistry [4]. Stille or Suzuki palladium-catalysed coupling reactions [5-10] are some of the most efficient methods for the preparation of 5-arylated 3-substituted thiophenes [11-14]. However, before these coupling reactions can be performed, an organometallic compound must be synthesized. In 1990, Ohta and co-workers described the Pd-catalysed direct arylation of thiophene derivatives by coupling reaction with aryl halides [15,16]. This is a highly powerful method for a greener access to a very broad range of arylated thiophenes [17-25]. The method is very attractive in terms of green chemistry, because its major by-products are not metal salts but a base associated to HX, and synthesis of an organometallic derivative can be avoided. However, for C3-substituted thiophenes, arylation generally occurred at the C2-position or gave mixtures of C2- and C5-arylated products [26-33]. The introduction of blocking groups at C2-position on thiophene derivatives in order to arylate regiospecifically the C5-positions had been reported (Figure 1).
Figure 1: Regioselectivity of the arylation of 3-substituted thiophenes.
Figure 1: Regioselectivity of the arylation of 3-substituted thiophenes.
In 2010, Fagnou et al. attached a 2-chloro-substituent to the thiophene ring to selectively perform a Pd-catalysed direct arylation of 3-hexylthiophene at the C5-position (Scheme 1, top) [34]. An ester moiety as blocking group at the C2-position of 3-substituted thiophene could also direct regioselectivity of Pd-catalysed direct arylation to the C5-position (Scheme 1, middle) [35]. Mori et al. also reported two examples of C5-arylation of 2-bromo-3-methylthiophene with aryl iodides as aryl sources with 5 mol % PdCl2(PPh3)2 catalyst and AgNO3–KF as the base in DMSO (Scheme 1, bottom) [36].
Scheme 1: Blocking groups allowing regioselective C5-arylation of thiophenes.
Scheme 1: Blocking groups allowing regioselective C5-arylation of thiophenes.
Herein, we wish to report on green conditions in terms of number of steps, base nature, use of a phosphine-free catalyst at low loading and a quite “atom economic” aryl source promoting such a C5-arylation using C3-substituted 2-bromothiophenes. We report i) that only 1 mol % of air-stable Pd(OAc)2 catalyst associated to KOAc promotes the regiospecific access to C5-arylated 2-bromothiophenes without cleavage of the thienyl C–Br bond, ii) on the reaction scope using a set of aryl bromides and 2-bromo-3-substituted thiophenes, iii) conditions allowing either the sequential C5-arylation followed by C2-arylation or C2-heteroarylation followed by C5-arylation of C3-substituted thiophenes.
Results and Discussion
Based on some of our previous results on Pd-catalysed direct arylation, for this study, DMA and KOAc were selected as the solvent and base [35]. The reaction of 2 equiv of 2-bromothiophene with 1 equiv of 4-bromonitrobenzene using 1 mol % of phosphine-free Pd(OAc)2 catalyst performed at 110 °C, only afforded the desired product 1 in a trace amount, but a complete conversion of 2-bromothiophene was observed, revealing the high reactivity of the thienyl C–Br bond under these conditions (Table 1, entry 1). Using a lower reaction temperature of 80 °C, and a reaction time of 15 h, the desired C5-arylated product 1 was formed in only 8% yield due again to the formation of several degradation products (Table 1, entry 2). Then, we examined the influence of the reaction time. After 2 or 4 h, higher yields of 1 (55% and 48%) were obtained, respectively; whereas, a very short reaction time of 0.5 h led to a lower yield of 27% due to the poor conversion of 4-bromonitrobenzene (Table 1, entries 3–6). The use of 0.5 mol % Pd(OAc)2 catalyst at 80 °C during 2 h also afforded 1 in a lower yield of 35%. Again, a large amount of 4-bromonitrobenzene was recovered (Table 1, entry 7). When CsOAc, NaOAc or K2CO3 were employed as bases instead of KOAc, in the presence of 1 mol % Pd(OAc)2 catalyst during 2 h, a partial conversion of 4-bromonitrobenzene was observed and 1 was isolated in 32–40% yield (Table 1, entries 8–10). It should be noted that in the presence of cyclopentyl methyl ether or diethyl carbonate as solvents, no formation of 1 was observed, and 4-bromonitrobenzene was recovered unreacted (Table 1, entries 11 and 12).
Table 1: Influence of the reaction conditions for the palladium-catalysed direct C5-arylation of 2-bromothiophene with 4-bromonitrobenzene.a
|
|
|||||
| Entry | Pd(OAc)2 (mol %) | Base | Temp (°C) | Time (h) | Yield in 1 (%) |
|---|---|---|---|---|---|
| 1 | 1 | KOAc | 110 | 15 | trace |
| 2 | 1 | KOAc | 80 | 15 | 8 |
| 3 | 1 | KOAc | 80 | 4 | 48 |
| 4 | 1 | KOAc | 80 | 2 | 55 |
| 5 | 1 | KOAc | 80 | 1 | 42 |
| 6 | 1 | KOAc | 80 | 0.5 | 27 |
| 7 | 0.5 | KOAc | 80 | 2 | 35 |
| 8 | 1 | CsOAc | 80 | 2 | 35 |
| 9 | 1 | NaOAc | 80 | 2 | 32 |
| 10 | 1 | K2CO3 | 80 | 2 | 40 |
| 11 | 1 | KOAc | 80 | 2 | 0b |
| 12 | 1 | KOAc | 80 | 2 | 0c |
aConditions: Pd(OAc)2, 2-bromothiophene (2 equiv), 4-bromonitrobenzene (1 equiv), base (2 equiv), DMA, isolated yields. bCyclopentyl methyl ether as solvent. cDiethyl carbonate as solvent.
Then, we studied the scope of this reaction using a set of aryl bromides and 2-bromothiophene derivatives, employing the most effective reaction conditions for C5-arylation of 2-bromothiophene (Table 1, entry 4: 1 mol % Pd(OAc)2, DMA, KOAc, 80 °C, 2 h) (Schemes 2–4). First, we investigated the reaction of 2-bromothiophene with 4-bromobenzonitrile, 4-bromobenzaldehyde and 4-bromo-2-(trifluoromethyl)nitrobenzene (Scheme 2). The expected coupling products 2–4 were obtained in moderate yields. On the other hand, with 4-bromoanisole as an electron-rich aryl bromide, the desired C5-arylated 2-bromothiophene could not be detected by GC–MS analysis of the crude mixture, and a large amount of unreacted 4-bromoanisole was recovered. Under these reaction conditions, the oxidative addition of 4-bromoanisole to palladium appears to be slower than the oxidative addition of 2-bromothiophene. Therefore, this procedure is limited to the use of electron-deficient aryl bromides. The reactivity of 2-bromofuran with 4-bromonitrobenzene was also investigated. Under the same reaction conditions, (1 mol % Pd(OAc)2, DMA, KOAc, 80 °C, 2 h) no formation of the desired 2-bromo-5-arylfuran derivative was observed.
Scheme 2: Reactivity of 2-bromothiophene with aryl bromides.
Scheme 2: Reactivity of 2-bromothiophene with aryl bromides.
The main interest to tolerate a C–Br bond at the C2-position on thiophene derivatives in the course of such couplings would be the regiospecific access to C5-arylated 3-substituted thiophenes, which cannot be obtained from 2-unsubstituted 3-substituted thiophenes such as 3-methylthiophene. Therefore, a set of aryl bromides was reacted with 2-bromo-3-methylthiophene, under these conditions (Scheme 3). Its reaction with aryl bromides para-substituted by nitro, cyano or formyl substituents gave the desired 5-arylated thiophenes 5–7 in 60–64% yields, without cleavage of the thienyl C–Br bond. Good yields of products 8 and 9 were also obtained from the meta-substituted aryl bromides, 3-bromobenzonitrile and 3-bromonitrobenzene. Again, a high yield of 85% of 10 was obtained with 4-bromo-2-(trifluoromethyl)nitrobenzene. Then, the reactivity of a set of ortho-substituted aryl bromides was examined. Bromobenzene containing nitro, nitrile or formyl ortho-substituents afforded the C5-arylated thiophenes 11–13 in 71–84% yields. Finally, 3-bromoquinoline and 3-bromopyrimidine were reacted with 2-bromo-3-methylthiophene affording 14 and 15 in 63% and 66% yields, respectively. The higher yields obtained for the arylation of 2-bromo-3-methylthiophene than with 2-bromothiophene are probably due to a slower oxidative addition of 2-bromo-3-methylthiophene to palladium which reduces the formation of side-products.
Scheme 3: Reactivity of 2-bromo-3-methylthiophene with (hetero)aryl bromides.
Scheme 3: Reactivity of 2-bromo-3-methylthiophene with (hetero)aryl bromides.
The reaction is not limited to the use of 2-bromo-3-methylthiophene. A 2-bromothiophene derivative bearing a CH2CO2Et substituent at C3 also provides regioselectively the desired C5-arylated thiophenes 16 and 17 in good yields; whereas, a lower yield of 18 was obtained for the coupling of 2-bromo-3-chlorothiophene with 4-bromobenzonitrile (Scheme 4).
Scheme 4: Reactivity of 3-substituted 2-bromothiophenes with aryl bromides.
Scheme 4: Reactivity of 3-substituted 2-bromothiophenes with aryl bromides.
Then, to demonstrate the synthetic potential of the thienyl bromo-substituent, product 1 was coupled with 2-methylthiophene in the presence of 1 mol % Pd(OAc)2 catalyst and KOAc as base (Scheme 5). The desired product 19 was obtained in 71% yield. Under the same conditions, a high yield of 91% in 20 was obtained from 2 and 2-methyl-4-ethylthiazole. These two reactions demonstrate that the sequential Pd-catalysed direct di-(hetero)arylation, using 2-bromothiophene as central unit, provides a powerful and simple access to non-symmetrically 2,5-di(hetero)arylated thiophene derivatives.
Scheme 5: 5-Heteroarylation of 2-aryl-5-bromothiophenes.
Scheme 5: 5-Heteroarylation of 2-aryl-5-bromothiophenes.
3-Substituted thiophene derivatives containing a heteroaryl unit at the C2-position and an aryl at C5 can also be obtained by direct heteroarylation at the C2-position of the C3-substituted 2-bromothiophene, followed by direct arylation at C5 (Scheme 6 and Scheme 7). First, we introduced imidazopyridinyl or thiazolyl groups at C2-position of 2-bromo-3-methylthiophene. In the presence of 1 mol % Pd(OAc)2 and KOAc as base at 150 °C the products 21–23 were obtained in 70–88% yields. In all cases, no C2-arylation of the 2-bromo-3-methylthiophene with itself to produce 5'-bromo-3,4'-dimethyl-2,2'-bithiophene was observed.
Scheme 6: 2-Heteroarylation of 2-bromo-3-methylthiophene.
Scheme 6: 2-Heteroarylation of 2-bromo-3-methylthiophene.
Then, from the C2-heteroarylated 3-methylthiophenes 21–23, a second direct arylation at position C5 allows to prepare the products 24–26 in 87–91% yields (Scheme 7).
Scheme 7: 5-Arylation of 2,3-disubstituted thiophenes.
Scheme 7: 5-Arylation of 2,3-disubstituted thiophenes.
The synthesis of 3-substituted thiophenes derivatives containing two different aryl groups at C2 and C5 positions via Suzuki coupling in the second step was also attempted (Scheme 8). The reaction of 5 with phenylboronic acid in the presence of only 1 mol % Pd(OAc)2 catalyst and K2CO3 as base gave 3-methyl-5-(4-nitrophenyl)-2-phenylthiophene (27) in 60% yield. A higher yield of 80% in 28 was obtained for the coupling of 16 with phenylboronic acid.
Scheme 8: 5-Arylation of 2-aryl-5-bromothiophenes.
Scheme 8: 5-Arylation of 2-aryl-5-bromothiophenes.
In order to further demonstrate that a bromo-substituent at C2-position of the thiophenes can be considered as a protecting group, we removed it via palladium-catalysed hydrogenolysis (Scheme 9). Treatment of 14 with 2 mol % Pd/C (10%) and trimethylamine in ethanol under hydrogen pressure, gave the desired debrominated product 29 in almost quantitative yield.
Scheme 9: Deprotection of 2-aryl-5-bromothiophene 14.
Scheme 9: Deprotection of 2-aryl-5-bromothiophene 14.
Conclusion
In summary, we report here that the use of a 2-bromo-substituent on thiophenes acts as a blocking group, allowing their regioselective Pd-catalysed C5-arylation even in the presence of aryl bromides as aryl sources. Only 1 mol % of phosphine-free air stable Pd(OAc)2 catalyst in the presence of KOAc as base promotes the C5-arylation of 2-bromothiophenes containing various C3-substituents with electron-deficient (hetero)aryl bromides. The sequential direct C5-arylation of 2-bromothiophenes followed either by a Suzuki coupling or a second direct arylation was found to allow the preparation of 2,5-di(hetero)arylated thiophenes bearing two different (hetero)aryl units. This method provides a convenient “greener” access to arylated thiophene derivatives as 1) it reduces the number of steps to prepare these compounds, 2) it employs the easily available Pd(OAc)2 catalyst and aryl bromides as aryl sources, and the inexpensive base KOAc, 3) it reduces the formation of wastes.
Supporting Information
| Supporting Information File 1: Procedures, 1H and 13C NMR data of all compounds. | ||
| Format: PDF | Size: 208.7 KB | Download |
References
-
Crouch, D. J.; Skabara, P. J.; Lohr, J. E.; McDouall, J. J. W.; Heeney, M.; McCulloch, I.; Sparrowe, D.; Shkunov, M.; Coles, S. J.; Horton, P. N.; Hursthouse, M. B. Chem. Mater. 2005, 17, 6567–6578. doi:10.1021/cm051563i
Return to citation in text: [1] -
Li, J.; Qiao, X.; Xiong, Y.; Li, H.; Zhu, D. Chem. Mater. 2014, 26, 5782–5788. doi:10.1021/cm502952u
Return to citation in text: [1] -
Kim, H. G.; Kang, B.; Ko, H.; Lee, J.; Shin, J.; Cho, K. Chem. Mater. 2015, 27, 829–838. doi:10.1021/cm503864u
Return to citation in text: [1] -
Li, C.-J.; Trost, B. M. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13197–13202. doi:10.1073/pnas.0804348105
Return to citation in text: [1] -
Li, J. J.; Gribble, G. W. Palladium in Heterocyclic Chemistry; Pergamon: Amsterdam, 2000.
Return to citation in text: [1] -
Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E., Ed.; Part III; Wiley-Interscience: New York, 2002; pp 213 ff.
Return to citation in text: [1] -
Ackermann, L. In Modern arylation methods; Ackermann, L., Ed.; Wiley Online Library, 2009.
Return to citation in text: [1] -
Brodnik, H.; Požgan, F.; Štefane, B. Org. Biomol. Chem. 2016, 14, 1969–1981. doi:10.1039/C5OB02364E
Return to citation in text: [1] -
Tùng, Đ. T.; Tuân, Đ. T.; Rasool, N.; Villinger, A.; Reinke, H.; Fischer, C.; Langer, P. Adv. Synth. Catal. 2009, 351, 1595–1609. doi:10.1002/adsc.200900044
Return to citation in text: [1] -
Toguem, S.-M. T.; Villinger, A.; Langer, P. Synlett 2010, 909–912. doi:10.1055/s-0029-1219380
Return to citation in text: [1] -
Hayashi, S.; Koizumi, T. Angew. Chem., Int. Ed. 2016, 55, 2701–2704. doi:10.1002/anie.201509319
Return to citation in text: [1] -
Hung, W.-I.; Liao, Y.-Y.; Lee, T.-H.; Ting, Y.-C.; Ni, J.-S.; Kao, W.-S.; Lin, J. T.; Wei, T.-C.; Yen, Y.-S. Chem. Commun. 2015, 51, 2152–2155. doi:10.1039/C4CC09294E
Return to citation in text: [1] -
Lincker, F.; Attias, A.-J.; Mathevet, F.; Heinrich, B.; Donnio, B.; Fave, J.-L.; Rannou, P.; Demadrille, R. Chem. Commun. 2012, 48, 3209–3211. doi:10.1039/c2cc17276c
Return to citation in text: [1] -
Pei, J.; Ni, J.; Zhou, X.-H.; Cao, X.-Y.; Lai, Y.-H. J. Org. Chem. 2002, 67, 4924–4936. doi:10.1021/jo011146z
Return to citation in text: [1] -
Ohta, A.; Akita, Y.; Ohkuwa, T.; Chiba, M.; Fukunaga, R.; Miyafuji, A.; Nakata, T.; Tani, N.; Aoyagi, Y. Heterocycles 1990, 31, 1951–1958. doi:10.3987/COM-90-5467
Return to citation in text: [1] -
Aoyagi, Y.; Inoue, A.; Koizumi, I.; Hashimoto, R.; Tokunaga, K.; Gohma, K.; Komatsu, J.; Sekine, K.; Miyafuji, A.; Kunoh, J.; Honma, R.; Akita, Y.; Ohta, A. Heterocycles 1992, 33, 257–272. doi:10.3987/COM-91-S29
Return to citation in text: [1] -
Li, B.-J.; Yang, S.-D.; Shi, Z.-J. Synlett 2008, 949–957. doi:10.1055/s-2008-1042907
Return to citation in text: [1] -
Roger, J.; Gottumukkala, A. L.; Doucet, H. ChemCatChem 2010, 2, 20–40. doi:10.1002/cctc.200900074
Return to citation in text: [1] -
Ackermann, L.; Vincente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996
Return to citation in text: [1] -
Bellina, F.; Rossi, R. Tetrahedron 2009, 65, 10269–10310. doi:10.1016/j.tet.2009.10.015
Return to citation in text: [1] -
Wencel-Delord, J.; Glorius, F. Nat. Chem. 2013, 5, 369–375. doi:10.1038/nchem.1607
Return to citation in text: [1] -
Kuzhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/C2CY20505J
Return to citation in text: [1] -
Rossi, R.; Bellina, F.; Lessi, M.; Manzini, C. Adv. Synth. Catal. 2014, 356, 17–117. doi:10.1002/adsc.201300922
Return to citation in text: [1] -
Yadav, M. R.; Rit, R. K.; Shankar, M.; Sahoo, A. K. Asian J. Org. Chem. 2015, 4, 846–864. doi:10.1002/ajoc.201500105
Return to citation in text: [1] -
Bheeter, C. B.; Chen, L.; Soulé, J.-F.; Doucet, H. Catal. Sci. Technol. 2016, 6, 2005–2049. doi:10.1039/C5CY02095F
Return to citation in text: [1] -
Lavenot, L.; Gozzi, C.; Ilg, K.; Orlova, I.; Penalva, V.; Lemaire, M. J. Organomet. Chem. 1998, 567, 49–55. doi:10.1016/S0022-328X(98)00667-6
Return to citation in text: [1] -
Glover, B.; Harvey, K. A.; Liu, B.; Sharp, M. J.; Tymoschenko, M. F. Org. Lett. 2003, 5, 301–304. doi:10.1021/ol027266q
Return to citation in text: [1] -
Dong, J. J.; Roy, D.; Jacob Roy, R.; Ionita, M.; Doucet, H. Synthesis 2011, 3530–3546. doi:10.1055/s-0030-1260213
Return to citation in text: [1] -
Chen, L.; Bruneau, C.; Dixneuf, P. H.; Doucet, H. Tetrahedron 2013, 69, 4381–4388. doi:10.1016/j.tet.2012.12.061
Return to citation in text: [1] -
Rene, O.; Fagnou, K. Org. Lett. 2010, 12, 2116–2119. doi:10.1021/ol1006136
Return to citation in text: [1] -
Dong, J. J.; Doucet, H. Eur. J. Org. Chem. 2010, 611–615. doi:10.1002/ejoc.200901213
Return to citation in text: [1] -
Forgione, P.; Brochu, M.-C.; St-Onge, M.; Thesen, K. H.; Bailey, M. D.; Bilodeau, F. J. Am. Chem. Soc. 2006, 128, 11350–11351. doi:10.1021/ja063511f
Return to citation in text: [1] -
Borghese, A.; Geldhof, G.; Antoine, L. Tetrahedron Lett. 2006, 47, 9249–9252. doi:10.1016/j.tetlet.2006.10.130
Return to citation in text: [1] -
Liégault, B.; Petrov, I.; Gorlesky, S. I.; Fagnou, K. J. Org. Chem. 2010, 75, 1047–1060. doi:10.1021/jo902515z
Return to citation in text: [1] -
Chen, L.; Bruneau, C.; Dixneuf, P. H.; Doucet, H. Green Chem. 2012, 14, 1111–1124. doi:10.1039/c2gc16460d
Return to citation in text: [1] [2] -
Kobayashi, K.; Sugie, A.; Takahashi, M.; Masui, K.; Mori, A. Org. Lett. 2005, 7, 5083–5085. doi:10.1021/ol052063y
Return to citation in text: [1]
| 1. | Crouch, D. J.; Skabara, P. J.; Lohr, J. E.; McDouall, J. J. W.; Heeney, M.; McCulloch, I.; Sparrowe, D.; Shkunov, M.; Coles, S. J.; Horton, P. N.; Hursthouse, M. B. Chem. Mater. 2005, 17, 6567–6578. doi:10.1021/cm051563i |
| 2. | Li, J.; Qiao, X.; Xiong, Y.; Li, H.; Zhu, D. Chem. Mater. 2014, 26, 5782–5788. doi:10.1021/cm502952u |
| 3. | Kim, H. G.; Kang, B.; Ko, H.; Lee, J.; Shin, J.; Cho, K. Chem. Mater. 2015, 27, 829–838. doi:10.1021/cm503864u |
| 15. | Ohta, A.; Akita, Y.; Ohkuwa, T.; Chiba, M.; Fukunaga, R.; Miyafuji, A.; Nakata, T.; Tani, N.; Aoyagi, Y. Heterocycles 1990, 31, 1951–1958. doi:10.3987/COM-90-5467 |
| 16. | Aoyagi, Y.; Inoue, A.; Koizumi, I.; Hashimoto, R.; Tokunaga, K.; Gohma, K.; Komatsu, J.; Sekine, K.; Miyafuji, A.; Kunoh, J.; Honma, R.; Akita, Y.; Ohta, A. Heterocycles 1992, 33, 257–272. doi:10.3987/COM-91-S29 |
| 11. | Hayashi, S.; Koizumi, T. Angew. Chem., Int. Ed. 2016, 55, 2701–2704. doi:10.1002/anie.201509319 |
| 12. | Hung, W.-I.; Liao, Y.-Y.; Lee, T.-H.; Ting, Y.-C.; Ni, J.-S.; Kao, W.-S.; Lin, J. T.; Wei, T.-C.; Yen, Y.-S. Chem. Commun. 2015, 51, 2152–2155. doi:10.1039/C4CC09294E |
| 13. | Lincker, F.; Attias, A.-J.; Mathevet, F.; Heinrich, B.; Donnio, B.; Fave, J.-L.; Rannou, P.; Demadrille, R. Chem. Commun. 2012, 48, 3209–3211. doi:10.1039/c2cc17276c |
| 14. | Pei, J.; Ni, J.; Zhou, X.-H.; Cao, X.-Y.; Lai, Y.-H. J. Org. Chem. 2002, 67, 4924–4936. doi:10.1021/jo011146z |
| 5. | Li, J. J.; Gribble, G. W. Palladium in Heterocyclic Chemistry; Pergamon: Amsterdam, 2000. |
| 6. | Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E., Ed.; Part III; Wiley-Interscience: New York, 2002; pp 213 ff. |
| 7. | Ackermann, L. In Modern arylation methods; Ackermann, L., Ed.; Wiley Online Library, 2009. |
| 8. | Brodnik, H.; Požgan, F.; Štefane, B. Org. Biomol. Chem. 2016, 14, 1969–1981. doi:10.1039/C5OB02364E |
| 9. | Tùng, Đ. T.; Tuân, Đ. T.; Rasool, N.; Villinger, A.; Reinke, H.; Fischer, C.; Langer, P. Adv. Synth. Catal. 2009, 351, 1595–1609. doi:10.1002/adsc.200900044 |
| 10. | Toguem, S.-M. T.; Villinger, A.; Langer, P. Synlett 2010, 909–912. doi:10.1055/s-0029-1219380 |
| 4. | Li, C.-J.; Trost, B. M. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13197–13202. doi:10.1073/pnas.0804348105 |
| 35. | Chen, L.; Bruneau, C.; Dixneuf, P. H.; Doucet, H. Green Chem. 2012, 14, 1111–1124. doi:10.1039/c2gc16460d |
| 35. | Chen, L.; Bruneau, C.; Dixneuf, P. H.; Doucet, H. Green Chem. 2012, 14, 1111–1124. doi:10.1039/c2gc16460d |
| 34. | Liégault, B.; Petrov, I.; Gorlesky, S. I.; Fagnou, K. J. Org. Chem. 2010, 75, 1047–1060. doi:10.1021/jo902515z |
| 26. | Lavenot, L.; Gozzi, C.; Ilg, K.; Orlova, I.; Penalva, V.; Lemaire, M. J. Organomet. Chem. 1998, 567, 49–55. doi:10.1016/S0022-328X(98)00667-6 |
| 27. | Glover, B.; Harvey, K. A.; Liu, B.; Sharp, M. J.; Tymoschenko, M. F. Org. Lett. 2003, 5, 301–304. doi:10.1021/ol027266q |
| 28. | Dong, J. J.; Roy, D.; Jacob Roy, R.; Ionita, M.; Doucet, H. Synthesis 2011, 3530–3546. doi:10.1055/s-0030-1260213 |
| 29. | Chen, L.; Bruneau, C.; Dixneuf, P. H.; Doucet, H. Tetrahedron 2013, 69, 4381–4388. doi:10.1016/j.tet.2012.12.061 |
| 30. | Rene, O.; Fagnou, K. Org. Lett. 2010, 12, 2116–2119. doi:10.1021/ol1006136 |
| 31. | Dong, J. J.; Doucet, H. Eur. J. Org. Chem. 2010, 611–615. doi:10.1002/ejoc.200901213 |
| 32. | Forgione, P.; Brochu, M.-C.; St-Onge, M.; Thesen, K. H.; Bailey, M. D.; Bilodeau, F. J. Am. Chem. Soc. 2006, 128, 11350–11351. doi:10.1021/ja063511f |
| 33. | Borghese, A.; Geldhof, G.; Antoine, L. Tetrahedron Lett. 2006, 47, 9249–9252. doi:10.1016/j.tetlet.2006.10.130 |
| 17. | Li, B.-J.; Yang, S.-D.; Shi, Z.-J. Synlett 2008, 949–957. doi:10.1055/s-2008-1042907 |
| 18. | Roger, J.; Gottumukkala, A. L.; Doucet, H. ChemCatChem 2010, 2, 20–40. doi:10.1002/cctc.200900074 |
| 19. | Ackermann, L.; Vincente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 20. | Bellina, F.; Rossi, R. Tetrahedron 2009, 65, 10269–10310. doi:10.1016/j.tet.2009.10.015 |
| 21. | Wencel-Delord, J.; Glorius, F. Nat. Chem. 2013, 5, 369–375. doi:10.1038/nchem.1607 |
| 22. | Kuzhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/C2CY20505J |
| 23. | Rossi, R.; Bellina, F.; Lessi, M.; Manzini, C. Adv. Synth. Catal. 2014, 356, 17–117. doi:10.1002/adsc.201300922 |
| 24. | Yadav, M. R.; Rit, R. K.; Shankar, M.; Sahoo, A. K. Asian J. Org. Chem. 2015, 4, 846–864. doi:10.1002/ajoc.201500105 |
| 25. | Bheeter, C. B.; Chen, L.; Soulé, J.-F.; Doucet, H. Catal. Sci. Technol. 2016, 6, 2005–2049. doi:10.1039/C5CY02095F |
| 36. | Kobayashi, K.; Sugie, A.; Takahashi, M.; Masui, K.; Mori, A. Org. Lett. 2005, 7, 5083–5085. doi:10.1021/ol052063y |
© 2016 Brahim et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)