Abstract
Selectivity in radical chain oligomerizations involving [1.1.1]propellane – i.e., to make [n]staffanes – has been notoriously challenging to control when n > 1 is desired. Herein, we report selective syntheses of SF5- and CF3SF4-containing [2]staffanes from SF5Cl and CF3SF4Cl, demonstrating cases whereby oligomerization is preferentially truncated after incorporation of two bicyclopentane (BCP) units. Synthetic and computational studies suggest this phenomenon can be attributed to alternating radical polarity matching. In addition, single-crystal X-ray diffraction (SC-XRD) data reveal structurally interesting features of the CF3SF4-containing [2]staffane in the solid state.
Graphical Abstract
Introduction
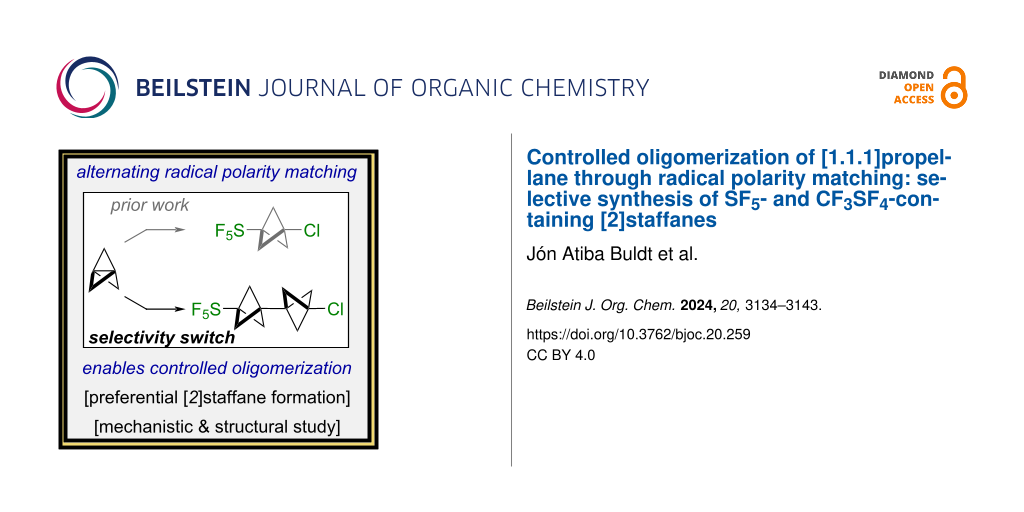
In various radical additions of X–Y across [1.1.1]propellane (1), functionalized oligomers known as [n]staffanes – with n > 1, where n denotes the number of individual [1.1.1]bicyclopentane (BCP) linkers – are often observed and swiftly devalorized as side-products [1]. However, targeted synthesis of functionalized [n]staffanes as rigid "molecular spacers" as proposed by Kaszynski and Michl [2-4] could facilitate new developments in nanotechnology [5], liquid crystal design [6-10], and the study of energy-transfer [11,12] or electron-transfer [13-17] processes. We also posit that lower-order [n]staffanes (i.e., n = 2 or 3) are potentially valuable C(sp3)-rich bioisosteres [18,19] that have been seemingly overlooked in the medicinal chemistry arena, in stark contrast to single BCP units over the past 12 years [20-24].
One plausible explanation for the paucity of applications of [n]staffanes in materials or biological settings is a synthetic accessibility issue. For instance, dimerization of substituted BCPs [25-27] or photochemical appendage of 1 onto an extant BCP [28-31] are relatively effective tactics for the selective assembly of certain [n]staffanes; the main caveat is that multiple synthetic steps are required. On the other hand, while a one-pot radical chain oligomerization is conceptually appealing, radical additions of X–Y across 1 in practice can be challenging to control and often lead to complex mixtures of functionalized [n]staffanes, n = 1–5 (Figure 1, top) [2,31-34]. Even though [n]staffanes are often separable by column chromatography, the yields for a single oligomer across a panoply of different transformations typically range from <1% to ≈30% when n > 1 is desired [35]. To the best of our knowledge, the assembly of functionalized [n]staffanes from 1 in high yield/selectivity and in one step via controlled radical oligomerization remains a synthetic challenge.
Figure 1: (Top) highlighting selectivity challenges in the synthesis of [n]staffanes using excess [1.1.1]propellane (1). (Bottom) selective synthesis of [2]staffanes bearing the SF5 (2) and CF3SF4 (3) groups (this work).
Figure 1: (Top) highlighting selectivity challenges in the synthesis of [n]staffanes using excess [1.1.1]prop...
Herein, we report proof-of-concept that our previous work on strain-release pentafluorosulfanylation of 1 [36] using SF5Cl (prepared in house [37] under mild oxidative fluorination conditions [38-44]) can be extended to the selective synthesis of the associated chloropentafluorosulfanylated [2]staffane (SF5-BCP-BCP-Cl, 2), based on alternating radical polarity matching in the chain-propagation steps (Figure 1, bottom) [45-47]. Density functional theory (DFT) calculations provide insight into our observed selectivity, and our hypothesis is bolstered by computation of relative bicyclopentyl radical philicities. In addition, we demonstrate that similar reaction conditions can be applied to the synthesis of the analogous CF3SF4-containing [2]staffane (CF3SF4-BCP-BCP-Cl, 3). Finally, we examined compound 3 by SC-XRD and found that it undergoes a phase transition as a function of rate of cooling; this highlights that the [2]staffanes synthesized during this study are also interesting from a fundamental structural standpoint.
Results and Discussion
Over the past few years, our group has begun to establish strain-release pentafluorosulfanylation as a viable strategy for C(sp3)–SF5 bond formation [35,48]. For instance, in 2022, we reported a method for chloropentafluorosulfanylation of [1.1.1]propellane, i.e., to make SF5-BCP-Cl (4), that ostensibly proceeds through a radical chain propagation mechanism [36]. Under optimized conditions, we obtained product 4 in 86% yield, and the corresponding [2]staffane – SF5-BCP-BCP-Cl (2) – was formed as a minor side-product in 7% yield. While our original goal was to suppress formation of 2, we later pondered whether preferential synthesis of compound 2 would also be possible. Accordingly, we began our screening process by systematically increasing the equivalents of [1.1.1]propellane (1) relative to SF5Cl and evaluating the impact on selectivity (Table 1).
Table 1: Effect of [1.1.1]propellane (1) equivalents relative to SF5Cl on selectivitya.
|
|
||||
| entry | 1 (equiv) | 4 (% yield)b | 2 (% yield)b | 4:2 |
| 1 | 1.0 | 49% | 4% | 12:1.0 |
| 2 | 2.0 | 74% | 22% | 3.4:1.0 |
| 3 | 3.0 | 44% | 29% | 1.5:1.0 |
| 4 | 4.0 | 54% | 40% | 1.3:1.0 |
| 5 | 6.0 | 48% | 45% | 1.1:1.0 |
| 6 | 8.0 | 43% | 53% | 1.0:1.3 |
| 7 | 10 | 32% | 51% | 1.0:1.6 |
| 8 | 20 | 24% | 72% | 1.0:3.0 |
| 9c | 6.0 | 30% | 63% (51%)d | 1.0:2.1 |
aA 0.1 M solution of SF5Cl in n-pentane (0.1 mmol) was added to a 0.8 M solution of [1.1.1]propellane in Et2O under Ar atmosphere and stirred at rt for 3 h. bYield determined by 19F NMR; cSF5Cl was added portion-wise. dIsolated yield.
A 0.1 M solution of SF5Cl in n-pentane was added to a 0.8 M solution of 1 in Et2O at room temperature, and the mixture was stirred for 3 hours prior to 19F NMR analysis. Upon increasing from 1.0 to 6.0 equiv of 1, we observed the 4:2 product ratio decreased dramatically from 12:1 to 1.1:1. Using 8.0 equiv of 1, the ratio flipped such that 2 became the major product (i.e., 4:2 = 1:1.3) and was formed in 53% yield. Interestingly, even with 8.0 equiv of 1, 96% of the material balance could be accounted for in the formation of these two products alone by 19F NMR. To the best of our knowledge, this is an exceptionally rare instance whereby oligomerization of 1 appears to be stunted beyond formation of the [2]staffane; only trace yields of putative higher-order staffanes (n > 2) were detected in the crude reaction mixture. Remarkably, using 20 equiv of 1, the observed 4:2 product ratio improved to 1:3, with 2 formed in 72% yield. In an even more extreme case, the [2]staffane still remained the major product formed in 66% yield when using 50 equiv of 1 (19F NMR signals of presumed higher-order staffanes became more apparent, though their combined yield still remained ≈18%).
Ultimately, we found adding SF5Cl portion-wise – to keep the "effective" equiv of 1 higher at any given moment – proved to be a suitable compromise to access 2 in 63% yield by 19F NMR (51% isolated) using 6.0 equiv of 1 (Table 1). We also demonstrated that this procedure can be performed on a 4.0 mmol scale (with respect to SF5Cl) to provide access to ≈0.5 g of 2 in 43% isolated yield. Additional details on reaction optimization can be found in Supporting Information File 1.
Upon increasing the equivalents of 1 during the screening process, we also found that irradiation with white LEDs was not necessary to boost product yields, as it was in our previously reported synthesis of 4 [36]. Both our laboratory [36] and the Qing laboratory [49] have previously observed that SF5Cl additions to 1 can proceed in the absence of light. Note that recent work from the laboratories of Cahard and Bizet [50] suggests that autoxidation of the ethereal solvent could serve as one possible explanation for initial formation of SF5 radicals in the absence of light to initiate a radical chain reaction. It is also well established that [1.1.1]propellane participates in radical-chain reactions (i.e., oligomerization) at room temperature in solution to form unsubstituted [n]staffanes.
The origin of this innately controlled oligomerization was then investigated through density functional theory (DFT) calculations. The free energy profile of the radical chain propagation sequence was computed at the PWPB95-D4/def2-QZVPP//PCM(Et2O)-ωB97X-D/def2-TZVP level of theory [51-58] (Figure 2). Following addition of an SF5 radical to 1 to form INT1, a Cl atom could be abstracted from SF5Cl via TS1 to form 4 or, alternatively, INT1 could be added to another equiv of 1 via TS2 to form INT2. Although formation of 4 is notably more thermodynamically favorable than INT2 (ΔΔG = −9.2 kcal/mol), a small difference in activation free energy is predicted (ΔΔG‡ = −1.4 kcal/mol). This, at least in part, provides an explanation as to how favoring the path to INT2 may be achieved in practice through increasing concentration of 1.
Figure 2: Computed free energy profile for the oligomerization of [1.1.1]propellane (1) following SF5 radical addition at PWPB95-D4/def2-QZVPP//PCM(Et2O)-ωB97X-D/def2-TZVP level of theory.
Figure 2: Computed free energy profile for the oligomerization of [1.1.1]propellane (1) following SF5 radical...
Subsequently, INT2 could abstract a Cl atom from SF5Cl via TS3 to form 2 or add to a third equiv of 1 through TS4, leading to INT3. Once again, Cl atom abstraction is thermodynamically (ΔΔG = −11.7 kcal/mol) and kinetically (ΔΔG‡ = −2.8 kcal/mol) favored. However, the ΔΔG‡ is notably greater in the second product-determining step than the first product-determining step, which is consistent with our experimental observation that 2 forms preferentially over further oligomerization.
For another point of comparison, we examined the reactivity of 1 with CF3SF4Cl. This reagent is known to behave comparably to SF5Cl in radical chain reactions [17,59-61] and can also be prepared conveniently in house [62]. In an analogous equivalents screen, we found that the 5:3 product ratio shifts from 7.7:1 using 1.0 equiv of 1 to 1:2.1 using 20 equiv of 1 (Table 2). In the latter scenario, 96% of the material balance could be accounted for in the formation of 5 and 3, indicating oligomerization is likewise stunted beyond incorporation of two BCP units. Also similar to pentafluorosulfanylation conditions, we found that adding CF3SF4Cl portion-wise to 6.0 equiv of 1 enables access to 3 in 60% yield by 19F NMR (53% isolated). Interestingly, we observed that aryl-SF4Cl compounds do not follow the same selectivity trend as SF5Cl and CF3SF4Cl additions, suggesting that the controlled oligomerization phenomenon is quite sensitive to changes in the fluorinated sulfur reagent scaffold (see Supporting Information File 1 for more details).
Table 2: Effect of [1.1.1]propellane (1) equivalents relative to CF3SF4Cl on selectivity.a
|
|
||||
| entry | 1 (equiv) | 5 (% yield)b | 3 (% yield)b | 5:3 |
| 1 | 1.0 | 80% | 10% | 7.7:1.0 |
| 2 | 2.0 | 71% | 21% | 3.4:1.0 |
| 3 | 3.0 | 65% | 31% | 2.1:1.0 |
| 4 | 4.0 | 55% | 40% | 1.4:1.0 |
| 5 | 6.0 | 49% | 40% | 1.2:1.0 |
| 6 | 8.0 | 39% | 46% | 1.0:1.2 |
| 7 | 10 | 33% | 62% | 1.0:1.9 |
| 8 | 20 | 31% | 65% | 1.0:2.1 |
| 9c | 6.0 | 35% | 60% (53%)d | 1.0:1.7 |
aA 0.1 M solution of CF3SF4Cl in n-pentane (0.03 mmol) was added to a 0.8 M solution of [1.1.1]propellane in Et2O under Ar atmosphere and stirred at rt for 3 h. bYield determined by 19F NMR. cCF3SF4Cl was added portion-wise. dIsolated yield.
This second instance of controlled oligomerization of 1 using CF3SF4Cl was also studied at the PWPB95-D4/def2-QZVPP//PCM(Et2O)-ωB97X-D/def2-TZVP level of theory (Figure 3). Addition of a CF3SF4 radical to 1 affords INT4, which can either abstract a Cl atom from CF3SF4Cl via TS5 to make 5 or add to another equiv of 1 via TS6 to access INT5. As anticipated, chlorination of the radical is thermodynamically favored over addition to 1 (ΔΔG = −10.7 kcal/mol). It is also predicted here that the free energy of activation is lower for chlorination, albeit only by 0.4 kcal/mol. This is consistent with the notion that the kinetic preference can be overcome by increasing the concentration of 1 relative to CF3SF4Cl. In the second product-determining step, Cl atom abstraction by INT5 via TS7 to make 3 is kinetically favorable over addition of a third equiv of 1 via TS8 to access INT6, although the preference is not as large as for formation of 2 (ΔΔG‡ = −1.3 kcal/mol).
Figure 3: Computed free energy profile for the oligomerization of [1.1.1]propellane (1) following CF3SF4 radical addition at the PWPB95-D4/def2-QZVPP//PCM(Et2O)-ωB97X-D/def2-TZVP level of theory.
Figure 3: Computed free energy profile for the oligomerization of [1.1.1]propellane (1) following CF3SF4 radi...
Thus, the predicted trend for both SF5Cl and CF3SF4Cl additions across 1 indicates a stronger preference for Cl atom abstraction over continued oligomerization in the second product-determining step than in the first – in line with our experimental observations. One possible explanation for this phenomenon is rooted in better radical polarity matching after incorporation of the second BCP unit [37,38]. That is, the carbon-centered radicals in both INT1 and INT4 are closer to strong electron-withdrawing groups than are the radical centers in INT2 and INT5, rendering INT1 and INT4 relatively more electrophilic. Inductive effects drop off steeply with distance, and it is also established that a substituent (or, e.g., a radical or cation) on the transannular carbon atom of a bicyclopentyl moiety can interact through space [35,63,64]. The consequence is ostensibly that more "nucleophilic" INT2 and INT5 are better matched for Cl atom abstraction from the "electrophilic" reagent (SF5Cl or CF3SF4Cl).
To test this hypothesis, we examined computed trends in various electronic parameters for the INT1–INT3 and the INT4–INT6 series (Table 3). For instance, across several charge models (i.e., Hirshfeld [65], NPA [66-68], and CHELPG [69]), the charge (q) on the carbon atom on which the radical is centered becomes more negative (or less positive) the farther it is from either the SF5 or CF3SF4 substituent, consistent with the notion that it becomes more nucleophilic. Moreover, the Δq is largest between the first two intermediates in both series – INT1 vs INT2 and INT3 vs INT4 – indicating that the most dramatic change in bicyclopentyl radical philicity would arise after incorporation of the second BCP unit.
Table 3: Key indices computed to compare reactivity. Partial charges (q) and condensed Fukui functions are evaluated at the reacting carbon or chlorine atom and are in units of elementary charge (e).a
|
|
||||||
| compound |
q(Hirshfled)
(e) |
q(NPA)
(e) |
q(CHELPG)
(e) |
f 0 (e)b | ω (eV)c | N (eV)d |
| 1 | −0.090 | −0.069 | −0.255 | 0.235 | 0.82 | 1.70 |
| SF5Cl | −0.055 | −0.160 | 0.030 | 0.534 | 2.48 | −0.74 |
| CF3SF4Cl | −0.061 | −0.150 | 0.056 | 0.527 | 2.52 | −0.66 |
| INT1 | −0.019 | 0.092 | −0.125 | 0.307 | 2.70 | 2.95 |
| INT2 | −0.053 | 0.073 | −0.196 | 0.347 | 1.78 | 3.58 |
| INT3 | −0.060 | 0.065 | −0.214 | 0.345 | 1.66 | 3.77 |
| INT4 | −0.019 | 0.092 | −0.144 | 0.301 | 2.74 | 2.97 |
| INT5 | −0.053 | 0.073 | −0.193 | 0.346 | 1.78 | 3.59 |
| INT6 | −0.060 | 0.064 | −0.214 | 0.345 | 1.65 | 3.77 |
aCalculations performed at the PCM(Et2O)-ωB97X-D/def2-TZVP level of theory. bRadical Fukui function. cElectrophilicity index. dNucleophilicity index.
In addition to charge models, we evaluated global reactivity indices (ω: electrophilicity index [70] and N: nucleophilicity index [71]) within the conceptual density functional theory (CDFT) framework [72-74]. The data show that BCP has a higher N value – thus stronger nucleophilic tendency – compared to both SF5Cl and CF3SF4Cl. Conversely, comparison of ω values shows significantly higher electrophilicity of SF5Cl and CF3SF4Cl compared to BCP. These results, coupled with decreasing ω and increasing N when more BCP units are incorporated, lend qualitative support to our radical polarity matching hypothesis. Moreover, assessment of radical Fukui functions (f0) [75] indicates that both SF5Cl and CF3SF4Cl are intrinsically more susceptible to radical attack than 1, which is consistent with the lower computed barriers for Cl atom abstraction in each case.
These computed trends also potentially account for the fact that selectivity for the [2]staffane (i.e., truncated oligomerization) using aryl-SF4Cl reagents was not observed. On the basis of CDFT results, a model aryl-SF4Cl compound (i.e., 5-chloropyrimidyl-SF4Cl) was determined to be significantly less "electrophilic" than SF5Cl or CF3SF4Cl, consistent with a reduction in the radical polarity matching effect (see Supporting Information File 1 for details). This was difficult to predict or rationalize based on calculation of free energies of activation alone. Overall, our results suggest that this alternating polarity matching effect is subtle and subject to mitigation yet can lead to desirable products if employed thoughtfully.
Lastly, following our synthetic and computational studies, accessing a CF3SF4-containing [2]staffane in good yield and for the first time created an opportunity for structural analysis. We previously reported and contextualized single-crystal X-ray diffraction (SC-XRD) data on 2 [36]; thus, we proceeded to grow crystals of 3 suitable for X-ray analysis through slow evaporation of ethyl acetate.
To our surprise, an initial measurement of 3 at 90 K revealed an unusually large unit cell (a = 7.14 Å, b = 21.38 Å, and c = 44.04 Å). Following structure solution and refinement [76], we found that 3 crystallizes in an orthorhombic space group P212121 with five symmetry independent moieties (Z' = 5) and with no solvent present in the unit cell as an inversion twin (Figure 4). After close examination of the model, we noticed that the c-axis was roughly divisible by five with a substructure of Z' = 1. This suggested that the Z' = 5 unit cell may be due to a phase transition caused by anisotropic contraction [77].
![[1860-5397-20-259-4]](/bjoc/content/figures/1860-5397-20-259-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: (A) The molecular structure of 3 at 90 K with 5 independent moieties in the asymmetric axis viewed along the b-axis. (B) The asymmetric unit of 3 at 90 K viewed along the c-axis. Thermal displacement ellipsoids depicted at 50% probability.
Figure 4: (A) The molecular structure of 3 at 90 K with 5 independent moieties in the asymmetric axis viewed ...
In fact, we confirmed that a phase transition had occurred following structure determination at 240 K [78,79]. The X-ray data revealed that 3 crystallizes in the centrosymmetric orthorhombic space group Pnma in the high temperature phase (HTP) with cell axes a = 21.56 Å, b = 8.95 Å, and c = 7.28 Å, in contrast to P212121 in the low temperature phase (LTP). Note that the b-axis is roughly 1/5 of the c-axis observed at 90 K (the axial rearrangement is due to the change in space group). To discern the approximate temperature of the phase transition, the unit cell was measured in 20 K increments upon cooling from 260 K down to 100 K; additional details are reported in Supporting Information File 1. Interestingly, the original LTP unit cell was not detected; instead, only the reduced cell observed in the HTP was found at all temperatures. However, after warming the same crystal of 3 back to room temperature, it was rapidly cooled to 100 K under a stream of N2 and the larger, disordered cell was observed once more [80]. (These observations also prompted us to measure a structure of 2 at 240 K; in this case, the unit cell is virtually identical at both high and low temperatures, indicating no phase transition had occurred – see Supporting Information File 1 for details.)
Accordingly, we gather that the rate of cooling plays an important role whereby rapid cooling effectively "shocks" the crystal of 3, compressing the unit cell isotropically, and ultimately leads to more disorder in the asymmetric unit [81]. This unexpected observation suggests that CF3SF4-containing [2]staffanes, in particular, warrant additional studies and may be of interest, e.g., in liquid crystal design.
Conclusion
Suppressing [n]staffane formation beyond n = 1 in radical chain reactions involving [1.1.1]propellane (1) tends to be more manageable than controlling oligomerization. However, under the right circumstances, alternating radical polarity matching throughout the chain propagation steps could be one way to theoretically "switch off" oligomerization beyond formation of a [2]staffane. Using this logic, our synthetic and computational study demonstrates that selective one-pot syntheses of [2]staffanes can be achieved when employing reagents that serve as radical sources of "extreme" electron-withdrawing groups (e.g., SF5 or CF3SF4), which impact relative philicities of the bicyclopentyl radical intermediates. Over the course of this study, we also found that the SF5- and CF3SF4-containing [2]staffanes reported herein are structurally interesting in their own right. Future work will examine potential applications of 2 and 3 and explore tactics for C–Cl bond functionalization.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, NMR spectra, computational details, and X-ray crystallographic experimental details. | ||
| Format: PDF | Size: 2.4 MB | Download |
| Supporting Information File 2: LTP X-ray crystal structure of compound 3 (2357079.cif), HTP X-ray crystal structure of compound 3 (2357080.cif) and X-ray crystal structure of compound 2 at 240 K (238115.cif). | ||
| Format: ZIP | Size: 365.1 KB | Download |
Acknowledgements
C.R.P. and Y.K. thank Dr. Nils Trapp (ETH Zürich) for helpful discussions. The authors also thank the UC Davis core NMR facility.
Funding
C.R.P. thanks the NIH/NIGMS (R35GM150861) and the University of California, Davis for financial support. W.Y.K thanks the Croucher Foundation for financial support through a doctoral scholarship. W.Y.K. and D.J.T. thank the NSF ACCESS program (CHE030089) for computational support. The authors also thank the NSF (CHE1531193) for the Dual Source X-ray Diffractometer.
Data Availability Statement
All experimental data that supports the findings of this study are available in the published article and/or the supporting information to this article; coordinates for computed structures are openly available in ioChem-BD at https://doi.org/10.19061/iochem-bd-6-384.
References
-
Shire, B. R.; Anderson, E. A. JACS Au 2023, 3, 1539–1553. doi:10.1021/jacsau.3c00014
Return to citation in text: [1] -
Kaszynski, P.; Friedli, A. C.; Michl, J. J. Am. Chem. Soc. 1992, 114, 601–620. doi:10.1021/ja00028a029
Return to citation in text: [1] [2] -
Levin, M. D.; Kaszynski, P.; Michl, J. Chem. Rev. 2000, 100, 169–234. doi:10.1021/cr990094z
Return to citation in text: [1] -
Dilmaç, A. M.; Spuling, E.; de Meijere, A.; Bräse, S. Angew. Chem., Int. Ed. 2017, 56, 5684–5718. doi:10.1002/anie.201603951
Return to citation in text: [1] -
Kaszynski, P.; Michl, J. J. Am. Chem. Soc. 1988, 110, 5225–5226. doi:10.1021/ja00223a070
Return to citation in text: [1] -
Friedli, A. C.; Lynch, V. M.; Kaszynski, P.; Michl, J. Acta Crystallogr., Sect. A: Found. Crystallogr. 1990, 46, 377–389. doi:10.1107/s0108768189014096
Return to citation in text: [1] -
Kaszynski, P.; Friedli, A. C.; McMurdie, N. D.; Michl, J. Mol. Cryst. Liq. Cryst. (1969-1991) 1990, 191, 193–197. doi:10.1080/00268949008038593
Return to citation in text: [1] -
Friberg, S. E.; Kayali, I.; Kaszynski, P.; Michl, J. Langmuir 1992, 8, 996–998. doi:10.1021/la00039a041
Return to citation in text: [1] -
Janecki, T.; Shi, S.; Kaszynski, P.; Michl, J. Collect. Czech. Chem. Commun. 1993, 58, 89–104. doi:10.1135/cccc19930089
Return to citation in text: [1] -
Messner, M.; Kozhushkov, S. I.; de Meijere, A. Eur. J. Org. Chem. 2000, 1137–1155. doi:10.1002/1099-0690(200004)2000:7<1137::aid-ejoc1137>3.0.co;2-2
Return to citation in text: [1] -
Zimmerman, H. E.; Goldman, T. D.; Hirzel, T. K.; Schmidt, S. P. J. Org. Chem. 1980, 45, 3933–3951. doi:10.1021/jo01308a001
Return to citation in text: [1] -
Obeng, Y. S.; Laing, M. E.; Friedli, A. C.; Yang, H. C.; Wang, D.; Thulstrup, E. W.; Bard, A. J.; Michl, J. J. Am. Chem. Soc. 1992, 114, 9943–9952. doi:10.1021/ja00051a029
Return to citation in text: [1] -
Joran, A. D.; Leland, B. A.; Geller, G. G.; Hopfield, J. J.; Dervan, P. B. J. Am. Chem. Soc. 1984, 106, 6090–6092. doi:10.1021/ja00332a062
Return to citation in text: [1] -
Leland, B. A.; Joran, A. D.; Felker, P. M.; Hopfield, J. J.; Zewail, A. H.; Dervan, P. B. J. Phys. Chem. 1985, 89, 5571–5573. doi:10.1021/j100272a002
Return to citation in text: [1] -
Murthy, G. S.; Hassenrück, K.; Lynch, V. M.; Michl, J. J. Am. Chem. Soc. 1989, 111, 7262–7264. doi:10.1021/ja00200a057
Return to citation in text: [1] -
Yang, H. C.; Magnera, T. F.; Lee, C.; Bard, A. J.; Michl, J. Langmuir 1992, 8, 2740–2746. doi:10.1021/la00047a026
Return to citation in text: [1] -
Mazières, S.; Raymond, M. K.; Raabe, G.; Prodi, A.; Michl, J. J. Am. Chem. Soc. 1997, 119, 6682–6683. doi:10.1021/ja971059h
Return to citation in text: [1] [2] -
Lovering, F.; Bikker, J.; Humblet, C. J. Med. Chem. 2009, 52, 6752–6756. doi:10.1021/jm901241e
Return to citation in text: [1] -
Tsien, J.; Hu, C.; Merchant, R. R.; Qin, T. Nat. Rev. Chem. 2024, 8, 605–627. doi:10.1038/s41570-024-00623-0
Return to citation in text: [1] -
Stepan, A. F.; Subramanyam, C.; Efremov, I. V.; Dutra, J. K.; O’Sullivan, T. J.; DiRico, K. J.; McDonald, W. S.; Won, A.; Dorff, P. H.; Nolan, C. E.; Becker, S. L.; Pustilnik, L. R.; Riddell, D. R.; Kauffman, G. W.; Kormos, B. L.; Zhang, L.; Lu, Y.; Capetta, S. H.; Green, M. E.; Karki, K.; Sibley, E.; Atchison, K. P.; Hallgren, A. J.; Oborski, C. E.; Robshaw, A. E.; Sneed, B.; O’Donnell, C. J. J. Med. Chem. 2012, 55, 3414–3424. doi:10.1021/jm300094u
Return to citation in text: [1] -
Locke, G. M.; Bernhard, S. S. R.; Senge, M. O. Chem. – Eur. J. 2019, 25, 4590–4647. doi:10.1002/chem.201804225
Return to citation in text: [1] -
Mykhailiuk, P. K. Org. Biomol. Chem. 2019, 17, 2839–2849. doi:10.1039/c8ob02812e
Return to citation in text: [1] -
Tse, E. G.; Houston, S. D.; Williams, C. M.; Savage, G. P.; Rendina, L. M.; Hallyburton, I.; Anderson, M.; Sharma, R.; Walker, G. S.; Obach, R. S.; Todd, M. H. J. Med. Chem. 2020, 63, 11585–11601. doi:10.1021/acs.jmedchem.0c00746
Return to citation in text: [1] -
Cuadros, S.; Goti, G.; Barison, G.; Raulli, A.; Bortolato, T.; Pelosi, G.; Costa, P.; Dell'Amico, L. Angew. Chem., Int. Ed. 2023, 62, e202303585. doi:10.1002/anie.202303585
Return to citation in text: [1] -
Rehm, J. D. D.; Ziemer, B.; Szeimies, G. Eur. J. Org. Chem. 2001, 1049–1052. doi:10.1002/1099-0690(200103)2001:6<1049::aid-ejoc1049>3.0.co;2-v
Return to citation in text: [1] -
Mazal, C.; Paraskos, A. J.; Michl, J. J. Org. Chem. 1998, 63, 2116–2119. doi:10.1021/jo971419j
Return to citation in text: [1] -
Bunz, U.; Szeimies, G. Tetrahedron Lett. 1990, 31, 651–652. doi:10.1016/s0040-4039(00)94592-1
Return to citation in text: [1] -
Blokhin, A. V.; Tyurekhodzhaeva, M. A.; Sadovaya, N. K.; Zefirov, N. S. Russ. Chem. Bull. 1989, 38, 1779. doi:10.1007/bf00956980
Return to citation in text: [1] -
Sadovaya, N. K.; Blokhin, A. V.; Tyurekhodzhaeva, M. A.; Grishin, Y. K.; Surmina, L. S.; Koz'min, A. S.; Zefirov, N. S. Russ. Chem. Bull. 1990, 39, 637–638. doi:10.1007/bf00959608
Return to citation in text: [1] -
Cheng, X.-Y.; Du, F.-S.; Li, Z.-C. J. Polym. Sci. (Hoboken, NJ, U. S.) 2023, 61, 472–481. doi:10.1002/pol.20220635
Return to citation in text: [1] -
Bunz, U.; Szeimies, G. Tetrahedron Lett. 1989, 30, 2087–2088. doi:10.1016/s0040-4039(01)93718-9
Return to citation in text: [1] [2] -
Bunz, U.; Polborn, K.; Wagner, H.-U.; Szeimies, G. Chem. Ber. 1988, 121, 1785–1790. doi:10.1002/cber.19881211014
Return to citation in text: [1] -
Sadovaya, N. K.; Blokhin, A. V.; Surmina, L. S.; Tyurekhodzhaeva, M. A.; Koz'min, A. S.; Zefirov, N. S. Russ. Chem. Bull. 1990, 39, 2224. doi:10.1007/bf01557749
Return to citation in text: [1] -
Wiberg, K. B.; Waddell, S. T. J. Am. Chem. Soc. 1990, 112, 2194–2216. doi:10.1021/ja00162a022
Return to citation in text: [1] -
Friedli, A. C.; Kaszynski, P.; Michl, J. Tetrahedron Lett. 1989, 30, 455–458. doi:10.1016/s0040-4039(00)95226-2
Return to citation in text: [1] [2] [3] -
Kraemer, Y.; Ghiazza, C.; Ragan, A. N.; Ni, S.; Lutz, S.; Neumann, E. K.; Fettinger, J. C.; Nöthling, N.; Goddard, R.; Cornella, J.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202211892. doi:10.1002/anie.202211892
Return to citation in text: [1] [2] [3] [4] [5] -
Shou, J.-Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2021, 60, 15271–15275. doi:10.1002/anie.202103606
Return to citation in text: [1] [2] -
Pitts, C. R.; Santschi, N.; Togni, A. Method For Preparing a Polyfluorinated Compound. WO Patent WO/2019/229103, Dec 5, 2019.
Return to citation in text: [1] [2] -
Pitts, C. R.; Bornemann, D.; Liebing, P.; Santschi, N.; Togni, A. Angew. Chem., Int. Ed. 2019, 58, 1950–1954. doi:10.1002/anie.201812356
Return to citation in text: [1] -
Häfliger, J.; Pitts, C. R.; Bornemann, D.; Käser, R.; Santschi, N.; Charpentier, J.; Otth, E.; Trapp, N.; Verel, R.; Lüthi, H. P.; Togni, A. Chem. Sci. 2019, 10, 7251–7259. doi:10.1039/c9sc02162k
Return to citation in text: [1] -
Bornemann, D.; Pitts, C. R.; Ziegler, C. J.; Pietrasiak, E.; Trapp, N.; Kueng, S.; Santschi, N.; Togni, A. Angew. Chem., Int. Ed. 2019, 58, 12604–12608. doi:10.1002/anie.201907359
Return to citation in text: [1] -
Brüning, F.; Pitts, C. R.; Kalim, J.; Bornemann, D.; Ghiazza, C.; de Montmollin, J.; Trapp, N.; Billard, T.; Togni, A. Angew. Chem., Int. Ed. 2019, 58, 18937–18941. doi:10.1002/anie.201910594
Return to citation in text: [1] -
Ragan, A. N.; Kraemer, Y.; Kong, W.-Y.; Prasad, S.; Tantillo, D. J.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202208046. doi:10.1002/anie.202208046
Return to citation in text: [1] -
Kraemer, Y.; Bergman, E. N.; Togni, A.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202205088. doi:10.1002/anie.202205088
Return to citation in text: [1] -
Tyurekhodzhaeva, M. A.; Bratkova, A. A.; Blokhin, A. V.; Brel, V. K.; Koz'min, A. S.; Zefirov, N. S. J. Fluorine Chem. 1991, 55, 237–240. doi:10.1016/s0022-1139(00)82351-9
Return to citation in text: [1] -
De Vleeschouwer, F.; Van Speybroeck, V.; Waroquier, M.; Geerlings, P.; De Proft, F. Org. Lett. 2007, 9, 2721–2724. doi:10.1021/ol071038k
Return to citation in text: [1] -
Domingo, L. R.; Pérez, P. Org. Biomol. Chem. 2013, 11, 4350–4358. doi:10.1039/c3ob40337h
Return to citation in text: [1] -
Kraemer, Y.; Buldt, J. A.; Kong, W.-Y.; Stephens, A. M.; Ragan, A. N.; Park, S.; Haidar, Z. C.; Patel, A. H.; Shey, R.; Dagan, R.; McLoughlin, C. P.; Fettinger, J. C.; Tantillo, D. J.; Pitts, C. R. Angew. Chem., Int. Ed. 2024, 63, e202319930. doi:10.1002/anie.202319930
Return to citation in text: [1] -
Zhao, X.; Shou, J.-Y.; Qing, F.-L. Sci. China: Chem. 2023, 66, 2871–2877. doi:10.1007/s11426-023-1715-2
Return to citation in text: [1] -
Nguyen, T. M.; Popek, L.; Matchavariani, D.; Blanchard, N.; Bizet, V.; Cahard, D. Org. Lett. 2024, 26, 365–369. doi:10.1021/acs.orglett.3c04043
Return to citation in text: [1] -
Goerigk, L.; Grimme, S. J. Chem. Theory Comput. 2011, 7, 291–309. doi:10.1021/ct100466k
Return to citation in text: [1] -
Caldeweyher, E.; Bannwarth, C.; Grimme, S. J. Chem. Phys. 2017, 147, 034112. doi:10.1063/1.4993215
Return to citation in text: [1] -
Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. J. Chem. Phys. 2019, 150, 154122. doi:10.1063/1.5090222
Return to citation in text: [1] -
Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. doi:10.1039/b508541a
Return to citation in text: [1] -
Cancès, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032–3041. doi:10.1063/1.474659
Return to citation in text: [1] -
Chai, J.-D.; Head-Gordon, M. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. doi:10.1039/b810189b
Return to citation in text: [1] -
Neese, F. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2022, 12, e1606. doi:10.1002/wcms.1606
Return to citation in text: [1] -
Gaussian 16, Revision. C.01; Gaussian, Inc.: Wallingford, CT, 2016.
Return to citation in text: [1] -
Ikeda, A.; Zhong, L.; Savoie, P. R.; von Hahmann, C. N.; Zheng, W.; Welch, J. T. Eur. J. Org. Chem. 2018, 772–780. doi:10.1002/ejoc.201701568
Return to citation in text: [1] -
Ikeda, A.; Capellan, A.; Welch, J. T. Org. Biomol. Chem. 2019, 17, 8079–8082. doi:10.1039/c9ob01797f
Return to citation in text: [1] -
Deng, M.; Wilde, M.; Welch, J. T. J. Org. Chem. 2023, 88, 11363–11366. doi:10.1021/acs.joc.3c01177
Return to citation in text: [1] -
Zhao, X.; Shou, J.-Y.; Newton, J. J.; Qing, F.-L. Org. Lett. 2022, 24, 8412–8416. doi:10.1021/acs.orglett.2c03540
Return to citation in text: [1] -
Wu, J. I.-C.; Schleyer, P. v. R. Pure Appl. Chem. 2013, 85, 921–940. doi:10.1351/pac-con-13-01-03
Return to citation in text: [1] -
Sterling, A. J.; Smith, R. C.; Anderson, E. A.; Duarte, F. J. Org. Chem. 2024, 89, 9979–9989. doi:10.1021/acs.joc.4c00857
Return to citation in text: [1] -
Hirshfeld, F. L. Theor. Chim. Acta 1977, 44, 129–138. doi:10.1007/bf00549096
Return to citation in text: [1] -
Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735–746. doi:10.1063/1.449486
Return to citation in text: [1] -
NBO 7.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2018.
Return to citation in text: [1] -
Glendening, E. D.; Landis, C. R.; Weinhold, F. J. Comput. Chem. 2019, 40, 2234–2241. doi:10.1002/jcc.25873
Return to citation in text: [1] -
Breneman, C. M.; Wiberg, K. B. J. Comput. Chem. 1990, 11, 361–373. doi:10.1002/jcc.540110311
Return to citation in text: [1] -
Parr, R. G.; Szentpály, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922–1924. doi:10.1021/ja983494x
Return to citation in text: [1] -
Domingo, L. R.; Chamorro, E.; Pérez, P. J. Org. Chem. 2008, 73, 4615–4624. doi:10.1021/jo800572a
Return to citation in text: [1] -
Lu, T.; Chen, Q. Realization of Conceptual Density Functional Theory and Information‐Theoretic Approach in Multiwfn Program. In Conceptual Density Functional Theory; Liu, S., Ed.; Wiley-VCH: Weinheim, Germany, 2022; Vol. 2, pp 631–647. doi:10.1002/9783527829941.ch31
Return to citation in text: [1] -
Geerlings, P.; De Proft, F.; Langenaeker, W. Chem. Rev. 2003, 103, 1793–1874. doi:10.1021/cr990029p
Return to citation in text: [1] -
Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580–592. doi:10.1002/jcc.22885
Return to citation in text: [1] -
Parr, R. G.; Yang, W. J. Am. Chem. Soc. 1984, 106, 4049–4050. doi:10.1021/ja00326a036
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218
Return to citation in text: [1] -
Stephenson, G. A. J. Pharm. Sci. 2006, 95, 821–827. doi:10.1002/jps.20442
Return to citation in text: [1] -
Chen, L.-Z.; Huang, D.-D.; Pan, Q.-J.; Ge, J.-Z. RSC Adv. 2015, 5, 13488–13494. doi:10.1039/c4ra12690d
Return to citation in text: [1] -
Tello, M. J.; Lopez-Echarri, A.; Zubillaga, J.; Ruiz-Larrea, I.; Zuniga, F. J.; Madariaga, G.; Gomez-Cuevas, A. J. Phys.: Condens. Matter 1994, 6, 6751–6760. doi:10.1088/0953-8984/6/34/007
Return to citation in text: [1] -
Izyumov, Y. A.; Syromyatnikov, V. N. Phase Transitions and Crystal Symmetry; Kluwer Academic Publishers: Dordrecht, Netherlands, 1990. doi:10.1007/978-94-009-1920-4
Return to citation in text: [1] -
Chattoraj, S.; Sun, C. C. Crystals 2023, 13, 270. doi:10.3390/cryst13020270
Return to citation in text: [1]
| 66. | Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735–746. doi:10.1063/1.449486 |
| 67. | NBO 7.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2018. |
| 68. | Glendening, E. D.; Landis, C. R.; Weinhold, F. J. Comput. Chem. 2019, 40, 2234–2241. doi:10.1002/jcc.25873 |
| 69. | Breneman, C. M.; Wiberg, K. B. J. Comput. Chem. 1990, 11, 361–373. doi:10.1002/jcc.540110311 |
| 1. | Shire, B. R.; Anderson, E. A. JACS Au 2023, 3, 1539–1553. doi:10.1021/jacsau.3c00014 |
| 11. | Zimmerman, H. E.; Goldman, T. D.; Hirzel, T. K.; Schmidt, S. P. J. Org. Chem. 1980, 45, 3933–3951. doi:10.1021/jo01308a001 |
| 12. | Obeng, Y. S.; Laing, M. E.; Friedli, A. C.; Yang, H. C.; Wang, D.; Thulstrup, E. W.; Bard, A. J.; Michl, J. J. Am. Chem. Soc. 1992, 114, 9943–9952. doi:10.1021/ja00051a029 |
| 38. | Pitts, C. R.; Santschi, N.; Togni, A. Method For Preparing a Polyfluorinated Compound. WO Patent WO/2019/229103, Dec 5, 2019. |
| 39. | Pitts, C. R.; Bornemann, D.; Liebing, P.; Santschi, N.; Togni, A. Angew. Chem., Int. Ed. 2019, 58, 1950–1954. doi:10.1002/anie.201812356 |
| 40. | Häfliger, J.; Pitts, C. R.; Bornemann, D.; Käser, R.; Santschi, N.; Charpentier, J.; Otth, E.; Trapp, N.; Verel, R.; Lüthi, H. P.; Togni, A. Chem. Sci. 2019, 10, 7251–7259. doi:10.1039/c9sc02162k |
| 41. | Bornemann, D.; Pitts, C. R.; Ziegler, C. J.; Pietrasiak, E.; Trapp, N.; Kueng, S.; Santschi, N.; Togni, A. Angew. Chem., Int. Ed. 2019, 58, 12604–12608. doi:10.1002/anie.201907359 |
| 42. | Brüning, F.; Pitts, C. R.; Kalim, J.; Bornemann, D.; Ghiazza, C.; de Montmollin, J.; Trapp, N.; Billard, T.; Togni, A. Angew. Chem., Int. Ed. 2019, 58, 18937–18941. doi:10.1002/anie.201910594 |
| 43. | Ragan, A. N.; Kraemer, Y.; Kong, W.-Y.; Prasad, S.; Tantillo, D. J.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202208046. doi:10.1002/anie.202208046 |
| 44. | Kraemer, Y.; Bergman, E. N.; Togni, A.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202205088. doi:10.1002/anie.202205088 |
| 6. | Friedli, A. C.; Lynch, V. M.; Kaszynski, P.; Michl, J. Acta Crystallogr., Sect. A: Found. Crystallogr. 1990, 46, 377–389. doi:10.1107/s0108768189014096 |
| 7. | Kaszynski, P.; Friedli, A. C.; McMurdie, N. D.; Michl, J. Mol. Cryst. Liq. Cryst. (1969-1991) 1990, 191, 193–197. doi:10.1080/00268949008038593 |
| 8. | Friberg, S. E.; Kayali, I.; Kaszynski, P.; Michl, J. Langmuir 1992, 8, 996–998. doi:10.1021/la00039a041 |
| 9. | Janecki, T.; Shi, S.; Kaszynski, P.; Michl, J. Collect. Czech. Chem. Commun. 1993, 58, 89–104. doi:10.1135/cccc19930089 |
| 10. | Messner, M.; Kozhushkov, S. I.; de Meijere, A. Eur. J. Org. Chem. 2000, 1137–1155. doi:10.1002/1099-0690(200004)2000:7<1137::aid-ejoc1137>3.0.co;2-2 |
| 45. | Tyurekhodzhaeva, M. A.; Bratkova, A. A.; Blokhin, A. V.; Brel, V. K.; Koz'min, A. S.; Zefirov, N. S. J. Fluorine Chem. 1991, 55, 237–240. doi:10.1016/s0022-1139(00)82351-9 |
| 46. | De Vleeschouwer, F.; Van Speybroeck, V.; Waroquier, M.; Geerlings, P.; De Proft, F. Org. Lett. 2007, 9, 2721–2724. doi:10.1021/ol071038k |
| 47. | Domingo, L. R.; Pérez, P. Org. Biomol. Chem. 2013, 11, 4350–4358. doi:10.1039/c3ob40337h |
| 78. | Chen, L.-Z.; Huang, D.-D.; Pan, Q.-J.; Ge, J.-Z. RSC Adv. 2015, 5, 13488–13494. doi:10.1039/c4ra12690d |
| 79. | Tello, M. J.; Lopez-Echarri, A.; Zubillaga, J.; Ruiz-Larrea, I.; Zuniga, F. J.; Madariaga, G.; Gomez-Cuevas, A. J. Phys.: Condens. Matter 1994, 6, 6751–6760. doi:10.1088/0953-8984/6/34/007 |
| 5. | Kaszynski, P.; Michl, J. J. Am. Chem. Soc. 1988, 110, 5225–5226. doi:10.1021/ja00223a070 |
| 36. | Kraemer, Y.; Ghiazza, C.; Ragan, A. N.; Ni, S.; Lutz, S.; Neumann, E. K.; Fettinger, J. C.; Nöthling, N.; Goddard, R.; Cornella, J.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202211892. doi:10.1002/anie.202211892 |
| 36. | Kraemer, Y.; Ghiazza, C.; Ragan, A. N.; Ni, S.; Lutz, S.; Neumann, E. K.; Fettinger, J. C.; Nöthling, N.; Goddard, R.; Cornella, J.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202211892. doi:10.1002/anie.202211892 |
| 2. | Kaszynski, P.; Friedli, A. C.; Michl, J. J. Am. Chem. Soc. 1992, 114, 601–620. doi:10.1021/ja00028a029 |
| 3. | Levin, M. D.; Kaszynski, P.; Michl, J. Chem. Rev. 2000, 100, 169–234. doi:10.1021/cr990094z |
| 4. | Dilmaç, A. M.; Spuling, E.; de Meijere, A.; Bräse, S. Angew. Chem., Int. Ed. 2017, 56, 5684–5718. doi:10.1002/anie.201603951 |
| 37. | Shou, J.-Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2021, 60, 15271–15275. doi:10.1002/anie.202103606 |
| 76. | Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218 |
| 25. | Rehm, J. D. D.; Ziemer, B.; Szeimies, G. Eur. J. Org. Chem. 2001, 1049–1052. doi:10.1002/1099-0690(200103)2001:6<1049::aid-ejoc1049>3.0.co;2-v |
| 26. | Mazal, C.; Paraskos, A. J.; Michl, J. J. Org. Chem. 1998, 63, 2116–2119. doi:10.1021/jo971419j |
| 27. | Bunz, U.; Szeimies, G. Tetrahedron Lett. 1990, 31, 651–652. doi:10.1016/s0040-4039(00)94592-1 |
| 2. | Kaszynski, P.; Friedli, A. C.; Michl, J. J. Am. Chem. Soc. 1992, 114, 601–620. doi:10.1021/ja00028a029 |
| 31. | Bunz, U.; Szeimies, G. Tetrahedron Lett. 1989, 30, 2087–2088. doi:10.1016/s0040-4039(01)93718-9 |
| 32. | Bunz, U.; Polborn, K.; Wagner, H.-U.; Szeimies, G. Chem. Ber. 1988, 121, 1785–1790. doi:10.1002/cber.19881211014 |
| 33. | Sadovaya, N. K.; Blokhin, A. V.; Surmina, L. S.; Tyurekhodzhaeva, M. A.; Koz'min, A. S.; Zefirov, N. S. Russ. Chem. Bull. 1990, 39, 2224. doi:10.1007/bf01557749 |
| 34. | Wiberg, K. B.; Waddell, S. T. J. Am. Chem. Soc. 1990, 112, 2194–2216. doi:10.1021/ja00162a022 |
| 72. | Lu, T.; Chen, Q. Realization of Conceptual Density Functional Theory and Information‐Theoretic Approach in Multiwfn Program. In Conceptual Density Functional Theory; Liu, S., Ed.; Wiley-VCH: Weinheim, Germany, 2022; Vol. 2, pp 631–647. doi:10.1002/9783527829941.ch31 |
| 73. | Geerlings, P.; De Proft, F.; Langenaeker, W. Chem. Rev. 2003, 103, 1793–1874. doi:10.1021/cr990029p |
| 74. | Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580–592. doi:10.1002/jcc.22885 |
| 20. | Stepan, A. F.; Subramanyam, C.; Efremov, I. V.; Dutra, J. K.; O’Sullivan, T. J.; DiRico, K. J.; McDonald, W. S.; Won, A.; Dorff, P. H.; Nolan, C. E.; Becker, S. L.; Pustilnik, L. R.; Riddell, D. R.; Kauffman, G. W.; Kormos, B. L.; Zhang, L.; Lu, Y.; Capetta, S. H.; Green, M. E.; Karki, K.; Sibley, E.; Atchison, K. P.; Hallgren, A. J.; Oborski, C. E.; Robshaw, A. E.; Sneed, B.; O’Donnell, C. J. J. Med. Chem. 2012, 55, 3414–3424. doi:10.1021/jm300094u |
| 21. | Locke, G. M.; Bernhard, S. S. R.; Senge, M. O. Chem. – Eur. J. 2019, 25, 4590–4647. doi:10.1002/chem.201804225 |
| 22. | Mykhailiuk, P. K. Org. Biomol. Chem. 2019, 17, 2839–2849. doi:10.1039/c8ob02812e |
| 23. | Tse, E. G.; Houston, S. D.; Williams, C. M.; Savage, G. P.; Rendina, L. M.; Hallyburton, I.; Anderson, M.; Sharma, R.; Walker, G. S.; Obach, R. S.; Todd, M. H. J. Med. Chem. 2020, 63, 11585–11601. doi:10.1021/acs.jmedchem.0c00746 |
| 24. | Cuadros, S.; Goti, G.; Barison, G.; Raulli, A.; Bortolato, T.; Pelosi, G.; Costa, P.; Dell'Amico, L. Angew. Chem., Int. Ed. 2023, 62, e202303585. doi:10.1002/anie.202303585 |
| 35. | Friedli, A. C.; Kaszynski, P.; Michl, J. Tetrahedron Lett. 1989, 30, 455–458. doi:10.1016/s0040-4039(00)95226-2 |
| 75. | Parr, R. G.; Yang, W. J. Am. Chem. Soc. 1984, 106, 4049–4050. doi:10.1021/ja00326a036 |
| 18. | Lovering, F.; Bikker, J.; Humblet, C. J. Med. Chem. 2009, 52, 6752–6756. doi:10.1021/jm901241e |
| 19. | Tsien, J.; Hu, C.; Merchant, R. R.; Qin, T. Nat. Rev. Chem. 2024, 8, 605–627. doi:10.1038/s41570-024-00623-0 |
| 70. | Parr, R. G.; Szentpály, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922–1924. doi:10.1021/ja983494x |
| 13. | Joran, A. D.; Leland, B. A.; Geller, G. G.; Hopfield, J. J.; Dervan, P. B. J. Am. Chem. Soc. 1984, 106, 6090–6092. doi:10.1021/ja00332a062 |
| 14. | Leland, B. A.; Joran, A. D.; Felker, P. M.; Hopfield, J. J.; Zewail, A. H.; Dervan, P. B. J. Phys. Chem. 1985, 89, 5571–5573. doi:10.1021/j100272a002 |
| 15. | Murthy, G. S.; Hassenrück, K.; Lynch, V. M.; Michl, J. J. Am. Chem. Soc. 1989, 111, 7262–7264. doi:10.1021/ja00200a057 |
| 16. | Yang, H. C.; Magnera, T. F.; Lee, C.; Bard, A. J.; Michl, J. Langmuir 1992, 8, 2740–2746. doi:10.1021/la00047a026 |
| 17. | Mazières, S.; Raymond, M. K.; Raabe, G.; Prodi, A.; Michl, J. J. Am. Chem. Soc. 1997, 119, 6682–6683. doi:10.1021/ja971059h |
| 28. | Blokhin, A. V.; Tyurekhodzhaeva, M. A.; Sadovaya, N. K.; Zefirov, N. S. Russ. Chem. Bull. 1989, 38, 1779. doi:10.1007/bf00956980 |
| 29. | Sadovaya, N. K.; Blokhin, A. V.; Tyurekhodzhaeva, M. A.; Grishin, Y. K.; Surmina, L. S.; Koz'min, A. S.; Zefirov, N. S. Russ. Chem. Bull. 1990, 39, 637–638. doi:10.1007/bf00959608 |
| 30. | Cheng, X.-Y.; Du, F.-S.; Li, Z.-C. J. Polym. Sci. (Hoboken, NJ, U. S.) 2023, 61, 472–481. doi:10.1002/pol.20220635 |
| 31. | Bunz, U.; Szeimies, G. Tetrahedron Lett. 1989, 30, 2087–2088. doi:10.1016/s0040-4039(01)93718-9 |
| 71. | Domingo, L. R.; Chamorro, E.; Pérez, P. J. Org. Chem. 2008, 73, 4615–4624. doi:10.1021/jo800572a |
| 36. | Kraemer, Y.; Ghiazza, C.; Ragan, A. N.; Ni, S.; Lutz, S.; Neumann, E. K.; Fettinger, J. C.; Nöthling, N.; Goddard, R.; Cornella, J.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202211892. doi:10.1002/anie.202211892 |
| 35. | Friedli, A. C.; Kaszynski, P.; Michl, J. Tetrahedron Lett. 1989, 30, 455–458. doi:10.1016/s0040-4039(00)95226-2 |
| 48. | Kraemer, Y.; Buldt, J. A.; Kong, W.-Y.; Stephens, A. M.; Ragan, A. N.; Park, S.; Haidar, Z. C.; Patel, A. H.; Shey, R.; Dagan, R.; McLoughlin, C. P.; Fettinger, J. C.; Tantillo, D. J.; Pitts, C. R. Angew. Chem., Int. Ed. 2024, 63, e202319930. doi:10.1002/anie.202319930 |
| 80. | Izyumov, Y. A.; Syromyatnikov, V. N. Phase Transitions and Crystal Symmetry; Kluwer Academic Publishers: Dordrecht, Netherlands, 1990. doi:10.1007/978-94-009-1920-4 |
| 36. | Kraemer, Y.; Ghiazza, C.; Ragan, A. N.; Ni, S.; Lutz, S.; Neumann, E. K.; Fettinger, J. C.; Nöthling, N.; Goddard, R.; Cornella, J.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202211892. doi:10.1002/anie.202211892 |
| 37. | Shou, J.-Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2021, 60, 15271–15275. doi:10.1002/anie.202103606 |
| 38. | Pitts, C. R.; Santschi, N.; Togni, A. Method For Preparing a Polyfluorinated Compound. WO Patent WO/2019/229103, Dec 5, 2019. |
| 35. | Friedli, A. C.; Kaszynski, P.; Michl, J. Tetrahedron Lett. 1989, 30, 455–458. doi:10.1016/s0040-4039(00)95226-2 |
| 63. | Wu, J. I.-C.; Schleyer, P. v. R. Pure Appl. Chem. 2013, 85, 921–940. doi:10.1351/pac-con-13-01-03 |
| 64. | Sterling, A. J.; Smith, R. C.; Anderson, E. A.; Duarte, F. J. Org. Chem. 2024, 89, 9979–9989. doi:10.1021/acs.joc.4c00857 |
| 17. | Mazières, S.; Raymond, M. K.; Raabe, G.; Prodi, A.; Michl, J. J. Am. Chem. Soc. 1997, 119, 6682–6683. doi:10.1021/ja971059h |
| 59. | Ikeda, A.; Zhong, L.; Savoie, P. R.; von Hahmann, C. N.; Zheng, W.; Welch, J. T. Eur. J. Org. Chem. 2018, 772–780. doi:10.1002/ejoc.201701568 |
| 60. | Ikeda, A.; Capellan, A.; Welch, J. T. Org. Biomol. Chem. 2019, 17, 8079–8082. doi:10.1039/c9ob01797f |
| 61. | Deng, M.; Wilde, M.; Welch, J. T. J. Org. Chem. 2023, 88, 11363–11366. doi:10.1021/acs.joc.3c01177 |
| 62. | Zhao, X.; Shou, J.-Y.; Newton, J. J.; Qing, F.-L. Org. Lett. 2022, 24, 8412–8416. doi:10.1021/acs.orglett.2c03540 |
| 50. | Nguyen, T. M.; Popek, L.; Matchavariani, D.; Blanchard, N.; Bizet, V.; Cahard, D. Org. Lett. 2024, 26, 365–369. doi:10.1021/acs.orglett.3c04043 |
| 51. | Goerigk, L.; Grimme, S. J. Chem. Theory Comput. 2011, 7, 291–309. doi:10.1021/ct100466k |
| 52. | Caldeweyher, E.; Bannwarth, C.; Grimme, S. J. Chem. Phys. 2017, 147, 034112. doi:10.1063/1.4993215 |
| 53. | Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. J. Chem. Phys. 2019, 150, 154122. doi:10.1063/1.5090222 |
| 54. | Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. doi:10.1039/b508541a |
| 55. | Cancès, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032–3041. doi:10.1063/1.474659 |
| 56. | Chai, J.-D.; Head-Gordon, M. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. doi:10.1039/b810189b |
| 57. | Neese, F. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2022, 12, e1606. doi:10.1002/wcms.1606 |
| 58. | Gaussian 16, Revision. C.01; Gaussian, Inc.: Wallingford, CT, 2016. |
| 36. | Kraemer, Y.; Ghiazza, C.; Ragan, A. N.; Ni, S.; Lutz, S.; Neumann, E. K.; Fettinger, J. C.; Nöthling, N.; Goddard, R.; Cornella, J.; Pitts, C. R. Angew. Chem., Int. Ed. 2022, 61, e202211892. doi:10.1002/anie.202211892 |
| 49. | Zhao, X.; Shou, J.-Y.; Qing, F.-L. Sci. China: Chem. 2023, 66, 2871–2877. doi:10.1007/s11426-023-1715-2 |
© 2024 Buldt et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.