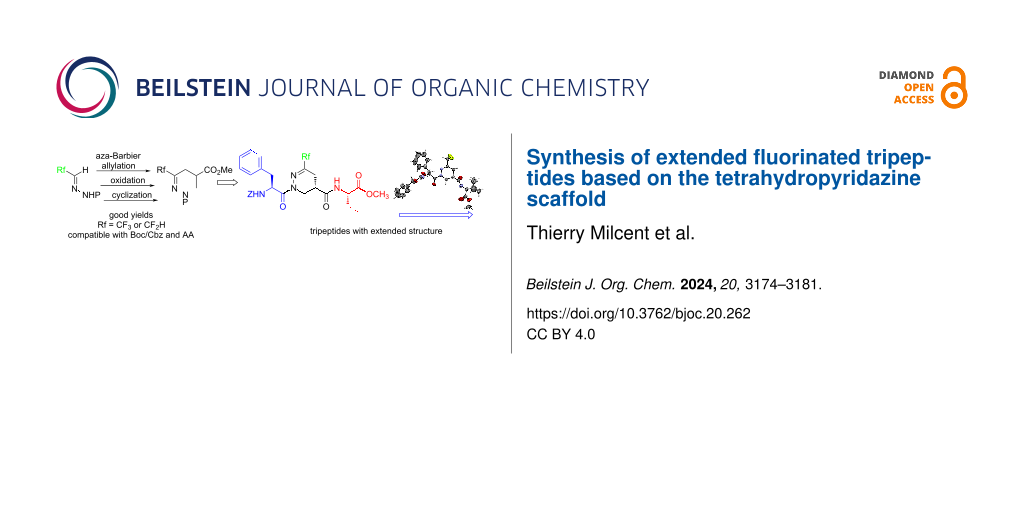
Abstract
The synthesis of tripeptides incorporating new fluorinated heterocyclic hydrazino acids, based on the tetrahydropyridazine scaffold is described. Starting from simple fluorinated hydrazones, these non-proteinogenic cyclic β-amino acids were easily prepared by a zinc-catalyzed aza-Barbier reaction followed by an intramolecular Michael addition. Preliminary conformational studies on tripeptides including this scaffold in the central position show an extended conformation in solution (NMR) and in the solid state (X-ray).
Graphical Abstract
Introduction
The synthesis of molecules capable of mimicking the various secondary structures and key functions of proteins is a major challenge in medicinal chemistry, especially in the fields of protein–protein interactions [1,2]. Accordingly, the incorporation of heterocyclic amino acids into peptides stabilizes their secondary structure and their metabolic stability, which is useful for numerous applications [3-5]. Indeed, the cyclic structure considerably reduces the number of possible rotational conformers, allowing a rational control of the 3D conformational space. Among these cyclic structures, the tetrahydropyridazines, six-atom nitrogenous heterocycles, are found in various bioactive molecules such as influenza virus neuraminidase inhibitors, GABA type A receptor modulators, and regulators of progesterone receptor or cannabinoid CB1 receptor antagonists (Figure 1) [6-9].
Figure 1: Examples of bioactive tetrahydropyridazine derivatives.
Figure 1: Examples of bioactive tetrahydropyridazine derivatives.
This tetrahydropyridazine scaffold is also found in numerous natural linear or cyclic peptides such as svetamycins or antrimycins as dehydropiperazic acid (Figure 2) [10].
Figure 2: Linear and cyclic peptides incorporating the dehydropiperazic acid moiety.
Figure 2: Linear and cyclic peptides incorporating the dehydropiperazic acid moiety.
Whereas many publications have been devoted to the synthesis and structure of piperazic acid derivatives (dehydro, chloro, hydroxy, …) [11,12], nothing is known about its β-analog (Figure 3), although β-amino acids have been shown to strongly modulate the structural, metabolic, and biological characteristics of peptides [13].
Figure 3: Piperazic acid and analogues and target trifluoro/difluoromethylated tetrahydropyridazine acids.
Figure 3: Piperazic acid and analogues and target trifluoro/difluoromethylated tetrahydropyridazine acids.
Finally, it is well known that fluorine is a very useful tool in medicinal chemistry as the incorporation of fluorinated groups (CF3 or CF2H) in organic molecules can modulate their physicochemical (pKa, lipophilicity), structural (additional hydrophobic and hydrogen-bond interactions), and biological properties (metabolic stability, membrane permeability) [14,15]. Alongside the very well-known CF3 group, the CF2H group has become an essential structural motif in medicinal chemistry due to its hydrogen-bond donor capacity, its lipophilic character, and as a bioisostere for alcohol, thiol, or amine groups [16-19]. Thus, the contribution of fluorinated compounds to pharmaceuticals has been crucial for more than half a century [20].
In this context and in our ongoing effort to synthesize fluorinated non-proteinogenic linear or cyclic β-amino acids [21,22], it appeared attractive to build fluorinated β-analogs of dehydropiperazic acid (Figure 3). This novel fluorinated amino acid 1 will combine the electronic and structural properties of the fluorinated groups (CF3 or CF2H) and the geometric constraints due to its partially unsaturated cycle, which could help in the design of peptidomimetics.
To our knowledge, only a few publications report the synthesis of tetrahydropyridazines with a carboxylic acid or ester substituent. Firstly, the group of Haupt reported the synthesis of ethyl esters of tetrahydromethylpyridazine in 20% yield in a mixture of methanol and water by the reaction of methylhydrazine with acetylene dicarboxylic esters through the formation of enhydrazine (Scheme 1a), [23]. Later, Tomilov et al. described the formation of tetrahydropyridazine 3,4,5,6-tetracarboxylic esters in 42% yield upon the decomposition in chloroform at 60 °C of methyl diazoacetate in the presence of pyridine and catalyzed by rhodium(II) acetate (Scheme 1b) [24,25]. More recently, an unusual [4 + 2]-cycloaddition reaction between electron-poor 1,2-diaza-1,3-dienes and electron-poor alkenes in refluxing acetonitrile was reported leading to various substituted tetrahydropyridazines in 17–78% yields (Scheme 1c) [26,27].
Scheme 1: Reported syntheses of tetrahydropyridazine ester derivatives.
Scheme 1: Reported syntheses of tetrahydropyridazine ester derivatives.
Nevertheless, these methods are neither relevant for the synthesis of 1 nor appropriate for peptide synthesis. Consequently, developing a simple and efficient methodology is still challenging. Our new strategy to synthesize these compounds is based on an aza-Barbier reaction on difluoro- or trifluoromethylated hydrazones. The thus obtained compounds will then be oxidized and cyclized in order to lead to the expected fluorinated tetrahydropyridazines (Figure 4).
Figure 4: Synthetic strategy to obtain fluorinated tetrahydropyridazines from difluoro- or trifluoromethylated hydrazones.
Figure 4: Synthetic strategy to obtain fluorinated tetrahydropyridazines from difluoro- or trifluoromethylate...
Results and Discussion
First, the difluoro- and trifluoromethylated hydrazones 3a–f were synthesized by condensing the corresponding hydrazide with the fluorinated aldehyde hemiacetal 2a or 2b (Scheme 2). Benzyl and tert-butyl carbazate (NH2-NHCbz/NH2-NHBoc) were chosen as starting materials in order to obtain final building blocks suitable for peptide synthesis. While the synthesis of compound 3a was already reported [28], compounds 3b–f are not described in the literature. All the fluorinated hydrazones were obtained in good yields and used directly in the next step without further purification (Scheme 2). In the case of Boc-protected hydrazones, the reaction must be carefully followed and reacted less than 2 hours in order to avoid the cleavage of the Boc group. The hydrazones 3e and 3f substituted by N-Cbz-ʟ-phenylalanine could easily be synthesized under the same reaction conditions starting from the corresponding hydrazide amino acid. Compounds 3e and 3f were obtained as a mixture of conformers (1:1 ratio) which is often observed with N-acylhydrazones [29-31]. Indeed, in theory, N-acylhydrazones can exhibit four isomers, two due to the E and Z isomers of the imine group (–N=CH–) and two due to the syn/anti-conformers of the amide bond (–NH-CO–). Experimentally, the E isomer is often more stable and so, predominant. The strong correlation between the NH and CH of the imine observed in 2D 1H-1H NOE experiments for the two conformers of hydrazones 3e and 3f (see Supporting Information File 1) is in accordance with the E stereoisomers. Furthermore, another correlation is observed for one conformer involving the NH of the imine on one side and the α-proton and the CH2 of the Cbz of the phenylalanine on the other. This correlation is present for one conformer (anti) and not for the other (syn), and this observation is similar for hydrazones 3e and 3f (see Supporting Information File 1). Based on these experimental data we can hypothesize the geometry of the two conformers of hydrazones 3e and 3f is E,syn and E,anti.
Scheme 2: Synthesis of fluorinated hydrazones 3a–f.
Scheme 2: Synthesis of fluorinated hydrazones 3a–f.
Then, hydrazones 3 were reacted with zinc and methyl 2-(bromomethyl)acrylate (4). This aza-Barbier reaction was performed in a biphasic medium (THF/aqueous solution of saturated NH4Cl) to avoid the formation of the α-methylene-γ-lactam obtained by intramolecular cyclization of the zinc amide on the ester function, as previously reported [32-35]. The corresponding adducts 5a–f were isolated with good yields from 66 to 88%. In the case of hydrazides 5e and 5f, the mixture of diastereomers (1:1 ratio) could not be separated at this stage. Although no stereoselectivity is observed, it can be noticed that the presence of an amino acid is compatible with the conditions of the reaction and did not interfere or significantly decrease the yield of the reaction (Scheme 3).
Scheme 3: Allylation of fluorinated hydrazones 3a–f to obtain 5a–f.
Scheme 3: Allylation of fluorinated hydrazones 3a–f to obtain 5a–f.
Then, the N-carboxylate hydrazides 5a–d were firstly oxidized with iodine in the presence of potassium carbonate to lead to the corresponding hydrazones 6a–d in good yields (69–80%). Surprisingly, these conditions were unsuitable for compounds 5e and 5f and led to the formation of numerous byproducts. Fortunately, the replacement of iodine with N-bromosuccinimide (NBS), previously reported for the oxidation of hydrazine [36], allowed the expected compounds 6e and 6f to be obtained in good yields. This methodology was applied to the previous hydrazides 5a–d giving the corresponding compounds 6a–d in similar yields. As expected, no isomerization occurred during the oxidation, leading exclusively to the imine and not the enamine. As for the hydrazones 3e and 3f, we assumed that the hydrazones 6e and 6f were obtained as a mixture of conformers, E,syn and E,anti (Scheme 4). Surprisingly, the ratio of each conformer differs for hydrazones 6e (ratio 77:23) and 6f (ratio 52:48).
Scheme 4: Oxidation of hydrazines 5a–f to obtain hydrazones 6a–f.
Scheme 4: Oxidation of hydrazines 5a–f to obtain hydrazones 6a–f.
The last cyclization step was based on an intramolecular Michael addition carried out in DMF and catalyzed by 10% of potassium carbonate. As previously observed [37], under these conditions, the 5-endo-trig cyclization was selective and the lactam resulting from the 5-exo-trig cyclization was not observed. Furthermore, in the case of compounds 7e and 7f, no competition with the NH of the phenylalanine was observed. The corresponding tetrahydropyridazines 7a–f were obtained in moderate to good yields (Scheme 5).
Scheme 5: Intramolecular cyclization of compounds 6a–f to obtain tetrahydropyridazines 7a–f.
Scheme 5: Intramolecular cyclization of compounds 6a–f to obtain tetrahydropyridazines 7a–f.
Concerning compounds 7e and 7f, each diastereomer of the 1:1 ratio mixture could be isolated after purification by flash silica chromatography.
With dipeptides 7e and 7f stereoisomerically pure, we next focused our attention on the preparation of novel peptidic structures to perform some conformational analyses. Starting from the methyl ester 7, each diastereomer was engaged in a classical sequence of saponification in the presence of LiOH, followed by a coupling reaction with ʟ-valine methyl ester hydrochloride, to give the corresponding four enantiomerically pure tripeptides 8 with satisfactory yields over two steps (Scheme 6).
Scheme 6: Preparation of tripeptides 8e, 8e’, 8f, and 8f’. Yields refer to the yield over 2 steps.
Scheme 6: Preparation of tripeptides 8e, 8e’, 8f, and 8f’. Yields refer to the yield over 2 steps.
The absolute configuration (S,S,S) of the isomer 8f was unambiguously assigned by X-ray crystallographic analysis (Figure 5).
Figure 5: X-ray diffraction of compound 8f.
Figure 5: X-ray diffraction of compound 8f.
Consequently, it was possible to assess the stereochemistry of the other diastereoisomer 8f’ and their precursors 7f and 7f’. On the other hand, considering the similarities of the 1H NMR spectra of the CF2H and CF3 analogs, by comparison, we could hypothesize the absolute configuration of compounds 7e and 7e’ and consequently of tripeptides 8e and 8e’ (see Supporting Information File 1).
Next, some preliminary conformational studies were performed. Firstly, the X-ray crystallographic analysis of compound 8f did not show any hydrogen-bond pattern and the global structure of the tripeptide is extended. Secondly, 2D 1H-1H NOE experiments of compound 8f confirmed this result. Indeed, no correlation was observed between the protons of the side chain of the phenylalanine and those of the valine, suggesting an extended structure in accordance with the X-ray structure. Furthermore, the 2D 19F,1H NOE experiments of compound 8f did not show any specific correlation between fluorine atoms and the protons of the amino acids (see Supporting Information File 1). Finally, the low chemical shifts of the amide and carbamate protons (6.4 ppm for NH of valine and 5.5 ppm for the NH of phenylalanine) indicate that they are not involved in hydrogen bonds. Furthermore, the 2D 1H-1H NOE NMR spectra and the chemical shifts of the NH protons of compounds 8f’, 8e, and 8e’ did not show any significant differences compared to 8f. Overall, the ability of the new fluorinated β-analogs of dehydropiperazic acid to act as β or γ-turn is excluded. Interestingly, this novel scaffold rather promotes extended structures.
Conclusion
To conclude, we have developed a new methodology to synthesize β-analogs of dehydropiperazic acid incorporating fluorinated groups. In order to improve the efficiency of this strategy, the control of the stereoselectivity of the intramolecular aza-Michael addition could be envisaged with various chiral catalysts in further studies. These heterocyclic hydrazino acids, when incorporated into the peptidic structure, appear to confer an extended conformation. These interesting feature results will be further confirmed by the insertion of these cyclic β-dehydropiperazic acids in longer peptide sequences.
Supporting Information
| Supporting Information File 1: Experimental procedures, product characterization, X-ray analysis and copies of NMR spectra. | ||
| Format: PDF | Size: 5.1 MB | Download |
Data Availability Statement
Additional research data generated and analyzed during this study is not shared.
References
-
Groß, A.; Hashimoto, C.; Sticht, H.; Eichler, J. Front. Bioeng. Biotechnol. 2016, 3, 211. doi:10.3389/fbioe.2015.00211
Return to citation in text: [1] -
Li Petri, G.; Di Martino, S.; De Rosa, M. J. Med. Chem. 2022, 65, 7438–7475. doi:10.1021/acs.jmedchem.2c00123
Return to citation in text: [1] -
Bąchor, U.; Mączyński, M. Molecules 2021, 26, 438. doi:10.3390/molecules26020438
Return to citation in text: [1] -
Miura, T.; Malla, T. R.; Brewitz, L.; Tumber, A.; Salah, E.; Lee, K. J.; Terasaka, N.; Owen, C. D.; Strain-Damerell, C.; Lukacik, P.; Walsh, M. A.; Kawamura, A.; Schofield, C. J.; Katoh, T.; Suga, H. Bull. Chem. Soc. Jpn. 2024, 97, uoae018. doi:10.1093/bulcsj/uoae018
Return to citation in text: [1] -
Liu, J.; Han, J.; Izawa, K.; Sato, T.; White, S.; Meanwell, N. A.; Soloshonok, V. A. Eur. J. Med. Chem. 2020, 208, 112736. doi:10.1016/j.ejmech.2020.112736
Return to citation in text: [1] -
Zhang, L.; Williams, M. A.; Mendel, D. B.; Escarpe, P. A.; Chen, X.; Wang, K.-Y.; Graves, B. J.; Lawton, G.; Kim, C. U. Bioorg. Med. Chem. Lett. 1999, 9, 1751–1756. doi:10.1016/s0960-894x(99)00280-2
Return to citation in text: [1] -
Rybczynski, P. J.; Combs, D. W.; Jacobs, K.; Shank, R. P.; Dubinsky, B. J. Med. Chem. 1999, 42, 2403–2408. doi:10.1021/jm9805889
Return to citation in text: [1] -
Combs, D. W.; Reese, K.; Phillips, A. J. Med. Chem. 1995, 38, 4878–4879. doi:10.1021/jm00025a003
Return to citation in text: [1] -
Lange, J. H. M.; den Hartog, A. P.; van der Neut, M. A. W.; van Vliet, B. J.; Kruse, C. G. Bioorg. Med. Chem. Lett. 2009, 19, 5675–5678. doi:10.1016/j.bmcl.2009.08.007
Return to citation in text: [1] -
Morgan, K. D.; Andersen, R. J.; Ryan, K. S. Nat. Prod. Rep. 2019, 36, 1628–1653. doi:10.1039/c8np00076j
Return to citation in text: [1] -
Handy, E. L.; Sello, J. K. Top. Heterocycl. Chem. 2015, 48, 97–124. doi:10.1007/7081_2015_185
Return to citation in text: [1] -
Ciufolini, M. A.; Xi, N. Chem. Soc. Rev. 1998, 27, 437–445. doi:10.1039/a827437z
Return to citation in text: [1] -
Cabrele, C.; Martinek, T. A.; Reiser, O.; Berlicki, Ł. J. Med. Chem. 2014, 57, 9718–9739. doi:10.1021/jm5010896
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f
Return to citation in text: [1] -
Zafrani, Y.; Yeffet, D.; Sod-Moriah, G.; Berliner, A.; Amir, D.; Marciano, D.; Gershonov, E.; Saphier, S. J. Med. Chem. 2017, 60, 797–804. doi:10.1021/acs.jmedchem.6b01691
Return to citation in text: [1] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Zafrani, Y.; Sod-Moriah, G.; Yeffet, D.; Berliner, A.; Amir, D.; Marciano, D.; Elias, S.; Katalan, S.; Ashkenazi, N.; Madmon, M.; Gershonov, E.; Saphier, S. J. Med. Chem. 2019, 62, 5628–5637. doi:10.1021/acs.jmedchem.9b00604
Return to citation in text: [1] -
Sessler, C. D.; Rahm, M.; Becker, S.; Goldberg, J. M.; Wang, F.; Lippard, S. J. J. Am. Chem. Soc. 2017, 139, 9325–9332. doi:10.1021/jacs.7b04457
Return to citation in text: [1] -
Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830
Return to citation in text: [1] -
Hao, J.; Milcent, T.; Retailleau, P.; Soloshonok, V. A.; Ongeri, S.; Crousse, B. Eur. J. Org. Chem. 2018, 3688–3692. doi:10.1002/ejoc.201800255
Return to citation in text: [1] -
Milcent, T.; Hao, J.; Kawada, K.; Soloshonok, V. A.; Ongeri, S.; Crousse, B. Eur. J. Org. Chem. 2014, 3072–3075. doi:10.1002/ejoc.201402078
Return to citation in text: [1] -
Sucrow, W.; Ellermann, K.-H.; Flörke, U.; Haupt, H.-J. Chem. Ber. 1988, 121, 2007–2012. doi:10.1002/cber.19881211117
Return to citation in text: [1] -
Tomilov, Y. V.; Platonov, D. N.; Averkiev, B. B.; Shulishov, E. V.; Nefedov, O. M. Russ. Chem. Bull. 2003, 52, 187–191. doi:10.1023/a:1022421020644
Return to citation in text: [1] -
Tomilov, Y. V.; Platonov, D. N.; Dorokhov, D. V.; Nefedov, O. M. Russ. Chem. Bull. 2005, 54, 1008–1012. doi:10.1007/s11172-005-0349-6
Return to citation in text: [1] -
D’Auria, M.; Racioppi, R.; Attanasi, O. A.; Mantellini, F. Synlett 2010, 1363–1366. doi:10.1055/s-0029-1219834
Return to citation in text: [1] -
Attanasi, O.; Bianchi, L.; D’Auria, M.; Mantellini, F.; Racioppi, R. Curr. Org. Synth. 2013, 10, 631–639. doi:10.2174/1570179411310040006
Return to citation in text: [1] -
Wang, J.; Shang, Y.; Zhao, X.; Cui, Z.; Li, Y.; Wang, K.-H.; Huang, D.; Hu, Y.; Wang, N.; Feng, L. Chem. Commun. 2023, 59, 14293–14296. doi:10.1039/d3cc03854h
Return to citation in text: [1] -
Munir, R.; Javid, N.; Zia-ur-Rehman, M.; Zaheer, M.; Huma, R.; Roohi, A.; Athar, M. M. Molecules 2021, 26, 4908. doi:10.3390/molecules26164908
Return to citation in text: [1] -
Socea, L.-I.; Barbuceanu, S.-F.; Pahontu, E. M.; Dumitru, A.-C.; Nitulescu, G. M.; Sfetea, R. C.; Apostol, T.-V. Molecules 2022, 27, 8719. doi:10.3390/molecules27248719
Return to citation in text: [1] -
Palla, G.; Predieri, G.; Domiano, P.; Vignali, C.; Turner, W. Tetrahedron 1986, 42, 3649–3654. doi:10.1016/s0040-4020(01)87332-4
Return to citation in text: [1] -
Xu, Y.; Huang, D.; Wang, K.-H.; Ma, J.; Su, Y.; Fu, Y.; Hu, Y. J. Org. Chem. 2015, 80, 12224–12233. doi:10.1021/acs.joc.5b02154
Return to citation in text: [1] -
Elaas, N. A.; Huang, D.; Wang, K.-H.; Su, Y.; Hu, Y. Heterocycl. Commun. 2018, 24, 159–163. doi:10.1515/hc-2017-0249
Return to citation in text: [1] -
Wang, J.; Huang, D.; Wang, K.-H.; Peng, X.; Su, Y.; Hu, Y.; Fu, Y. Org. Biomol. Chem. 2016, 14, 9533–9542. doi:10.1039/c6ob01487a
Return to citation in text: [1] -
Du, G.; Huang, D.; Wang, K.-H.; Chen, X.; Xu, Y.; Ma, J.; Su, Y.; Fu, Y.; Hu, Y. Org. Biomol. Chem. 2016, 14, 1492–1500. doi:10.1039/c5ob02260f
Return to citation in text: [1] -
Wang, Y.; Wang, K.-H.; Su, Y.; Yang, Z.; Wen, L.; Liu, L.; Wang, J.; Huang, D.; Hu, Y. J. Org. Chem. 2018, 83, 939–950. doi:10.1021/acs.joc.7b02934
Return to citation in text: [1] -
Ichikawa, J.; Lapointe, G.; Iwai, Y. Chem. Commun. 2007, 2698–2700. doi:10.1039/b618251h
Return to citation in text: [1]
| 36. | Wang, Y.; Wang, K.-H.; Su, Y.; Yang, Z.; Wen, L.; Liu, L.; Wang, J.; Huang, D.; Hu, Y. J. Org. Chem. 2018, 83, 939–950. doi:10.1021/acs.joc.7b02934 |
| 37. | Ichikawa, J.; Lapointe, G.; Iwai, Y. Chem. Commun. 2007, 2698–2700. doi:10.1039/b618251h |
| 1. | Groß, A.; Hashimoto, C.; Sticht, H.; Eichler, J. Front. Bioeng. Biotechnol. 2016, 3, 211. doi:10.3389/fbioe.2015.00211 |
| 2. | Li Petri, G.; Di Martino, S.; De Rosa, M. J. Med. Chem. 2022, 65, 7438–7475. doi:10.1021/acs.jmedchem.2c00123 |
| 11. | Handy, E. L.; Sello, J. K. Top. Heterocycl. Chem. 2015, 48, 97–124. doi:10.1007/7081_2015_185 |
| 12. | Ciufolini, M. A.; Xi, N. Chem. Soc. Rev. 1998, 27, 437–445. doi:10.1039/a827437z |
| 29. | Munir, R.; Javid, N.; Zia-ur-Rehman, M.; Zaheer, M.; Huma, R.; Roohi, A.; Athar, M. M. Molecules 2021, 26, 4908. doi:10.3390/molecules26164908 |
| 30. | Socea, L.-I.; Barbuceanu, S.-F.; Pahontu, E. M.; Dumitru, A.-C.; Nitulescu, G. M.; Sfetea, R. C.; Apostol, T.-V. Molecules 2022, 27, 8719. doi:10.3390/molecules27248719 |
| 31. | Palla, G.; Predieri, G.; Domiano, P.; Vignali, C.; Turner, W. Tetrahedron 1986, 42, 3649–3654. doi:10.1016/s0040-4020(01)87332-4 |
| 10. | Morgan, K. D.; Andersen, R. J.; Ryan, K. S. Nat. Prod. Rep. 2019, 36, 1628–1653. doi:10.1039/c8np00076j |
| 32. | Xu, Y.; Huang, D.; Wang, K.-H.; Ma, J.; Su, Y.; Fu, Y.; Hu, Y. J. Org. Chem. 2015, 80, 12224–12233. doi:10.1021/acs.joc.5b02154 |
| 33. | Elaas, N. A.; Huang, D.; Wang, K.-H.; Su, Y.; Hu, Y. Heterocycl. Commun. 2018, 24, 159–163. doi:10.1515/hc-2017-0249 |
| 34. | Wang, J.; Huang, D.; Wang, K.-H.; Peng, X.; Su, Y.; Hu, Y.; Fu, Y. Org. Biomol. Chem. 2016, 14, 9533–9542. doi:10.1039/c6ob01487a |
| 35. | Du, G.; Huang, D.; Wang, K.-H.; Chen, X.; Xu, Y.; Ma, J.; Su, Y.; Fu, Y.; Hu, Y. Org. Biomol. Chem. 2016, 14, 1492–1500. doi:10.1039/c5ob02260f |
| 6. | Zhang, L.; Williams, M. A.; Mendel, D. B.; Escarpe, P. A.; Chen, X.; Wang, K.-Y.; Graves, B. J.; Lawton, G.; Kim, C. U. Bioorg. Med. Chem. Lett. 1999, 9, 1751–1756. doi:10.1016/s0960-894x(99)00280-2 |
| 7. | Rybczynski, P. J.; Combs, D. W.; Jacobs, K.; Shank, R. P.; Dubinsky, B. J. Med. Chem. 1999, 42, 2403–2408. doi:10.1021/jm9805889 |
| 8. | Combs, D. W.; Reese, K.; Phillips, A. J. Med. Chem. 1995, 38, 4878–4879. doi:10.1021/jm00025a003 |
| 9. | Lange, J. H. M.; den Hartog, A. P.; van der Neut, M. A. W.; van Vliet, B. J.; Kruse, C. G. Bioorg. Med. Chem. Lett. 2009, 19, 5675–5678. doi:10.1016/j.bmcl.2009.08.007 |
| 26. | D’Auria, M.; Racioppi, R.; Attanasi, O. A.; Mantellini, F. Synlett 2010, 1363–1366. doi:10.1055/s-0029-1219834 |
| 27. | Attanasi, O.; Bianchi, L.; D’Auria, M.; Mantellini, F.; Racioppi, R. Curr. Org. Synth. 2013, 10, 631–639. doi:10.2174/1570179411310040006 |
| 3. | Bąchor, U.; Mączyński, M. Molecules 2021, 26, 438. doi:10.3390/molecules26020438 |
| 4. | Miura, T.; Malla, T. R.; Brewitz, L.; Tumber, A.; Salah, E.; Lee, K. J.; Terasaka, N.; Owen, C. D.; Strain-Damerell, C.; Lukacik, P.; Walsh, M. A.; Kawamura, A.; Schofield, C. J.; Katoh, T.; Suga, H. Bull. Chem. Soc. Jpn. 2024, 97, uoae018. doi:10.1093/bulcsj/uoae018 |
| 5. | Liu, J.; Han, J.; Izawa, K.; Sato, T.; White, S.; Meanwell, N. A.; Soloshonok, V. A. Eur. J. Med. Chem. 2020, 208, 112736. doi:10.1016/j.ejmech.2020.112736 |
| 28. | Wang, J.; Shang, Y.; Zhao, X.; Cui, Z.; Li, Y.; Wang, K.-H.; Huang, D.; Hu, Y.; Wang, N.; Feng, L. Chem. Commun. 2023, 59, 14293–14296. doi:10.1039/d3cc03854h |
| 20. | Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830 |
| 23. | Sucrow, W.; Ellermann, K.-H.; Flörke, U.; Haupt, H.-J. Chem. Ber. 1988, 121, 2007–2012. doi:10.1002/cber.19881211117 |
| 16. | Zafrani, Y.; Yeffet, D.; Sod-Moriah, G.; Berliner, A.; Amir, D.; Marciano, D.; Gershonov, E.; Saphier, S. J. Med. Chem. 2017, 60, 797–804. doi:10.1021/acs.jmedchem.6b01691 |
| 17. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 18. | Zafrani, Y.; Sod-Moriah, G.; Yeffet, D.; Berliner, A.; Amir, D.; Marciano, D.; Elias, S.; Katalan, S.; Ashkenazi, N.; Madmon, M.; Gershonov, E.; Saphier, S. J. Med. Chem. 2019, 62, 5628–5637. doi:10.1021/acs.jmedchem.9b00604 |
| 19. | Sessler, C. D.; Rahm, M.; Becker, S.; Goldberg, J. M.; Wang, F.; Lippard, S. J. J. Am. Chem. Soc. 2017, 139, 9325–9332. doi:10.1021/jacs.7b04457 |
| 24. | Tomilov, Y. V.; Platonov, D. N.; Averkiev, B. B.; Shulishov, E. V.; Nefedov, O. M. Russ. Chem. Bull. 2003, 52, 187–191. doi:10.1023/a:1022421020644 |
| 25. | Tomilov, Y. V.; Platonov, D. N.; Dorokhov, D. V.; Nefedov, O. M. Russ. Chem. Bull. 2005, 54, 1008–1012. doi:10.1007/s11172-005-0349-6 |
| 14. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 15. | Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f |
| 13. | Cabrele, C.; Martinek, T. A.; Reiser, O.; Berlicki, Ł. J. Med. Chem. 2014, 57, 9718–9739. doi:10.1021/jm5010896 |
| 21. | Hao, J.; Milcent, T.; Retailleau, P.; Soloshonok, V. A.; Ongeri, S.; Crousse, B. Eur. J. Org. Chem. 2018, 3688–3692. doi:10.1002/ejoc.201800255 |
| 22. | Milcent, T.; Hao, J.; Kawada, K.; Soloshonok, V. A.; Ongeri, S.; Crousse, B. Eur. J. Org. Chem. 2014, 3072–3075. doi:10.1002/ejoc.201402078 |
© 2024 Milcent et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.